The scientific complexity and economic cost of developing new cancer therapies demand a level of collaboration and sharing that takes both industry and academia well beyond their comfort zones. EORTC head Denis Lacombe believes he has the passion and the vision to help make it happen.
 It is 22 years since Denis Lacombe first stepped into the Brussels offices of the European Organisation for Research and Treatment of Cancer (EORTC) on a fellowship. Today, speaking to him two months after his appointment as its Director General, he can still barely believe he has worked his way up to lead the organisation – the only European non-profit body carrying out international multidisciplinary research for all types of cancer.
It is 22 years since Denis Lacombe first stepped into the Brussels offices of the European Organisation for Research and Treatment of Cancer (EORTC) on a fellowship. Today, speaking to him two months after his appointment as its Director General, he can still barely believe he has worked his way up to lead the organisation – the only European non-profit body carrying out international multidisciplinary research for all types of cancer.
But though Lacombe, a Frenchman with a research background in pharmacology and pharmacokinetics, clearly has his feet planted in EORTC’s history and values, what he really wants to talk about is radical change.
“To be honest, the environment has changed so much, and there is so much potential in the organisation, that I feel that I’ve just arrived,” he says.
Today, says Lacombe, we are witnessing nothing less than a revolution in the way cancer treatments are developed and researched. And he sees EORTC at the vanguard of change in Europe, leading the way against stifling regulations, professional silos and antiquated trials processes, and towards a new era of personalised medicines tested as early as possible on those who need them most.
“The reason I am still here is that I truly believe in our mission,” he says. “I believe in the multidisciplinary team, in partnerships, in what we can do for patients. Most commercial clinical trials are purely drug-orientated, but what we do is independent and genuinely patient-centred, also addressing surgery and radiation oncology. The forms and methods of clinical trials have evolved over the years, but we have always had the capacity to ask the questions that patients want answering, and to follow patients long-term.”
As he sets out his credo at the start of our interview, he consults notes he has diligently prepared for our meeting. My questions about the challenges the research world faces – particularly self-interest and insularity in academia and industry – are politely acknowledged as valid, but shifted towards his vision of change and opportunity in Europe, led by EORTC. It rings of genuine enthusiasm rather than corporate PR.
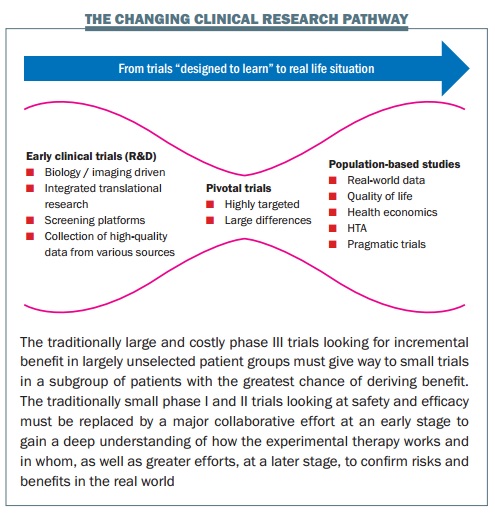
At the centre of his excitement is the concept of a child’s toy – the diabolo, an hourglass shaped cylinder, controlled on a string between two sticks. He sketches it on a piece of paper in front of him. This, he explains, is the new shape of clinical research in cancer.
Such is the diversity of disease and drugs now designed to target specific types and people that the classic triangular model of treatment development – with more and more resources being poured in as drugs move from phase I to phase III trials – is no longer fit for purpose, says Lacombe.
Historically, EORTC concentrated on large phase II and III clinical trials. But today, he explains, resources are moving “upstream” to early clinical trials involving tissue characterisation, imaging, screening platforms, collection of high-quality data. This should allow for much smaller pivotal trials (trials aimed at changing practice, represented by the narrow centre of the diabolo), which should be done with highly targeted groups, where the benefits are likely to outweigh the risks. Then the diabolo shape opens up again to represent increasing efforts over the longer term to establish the true value of the treatment in real-life settings.
And here new models are emerging for post-marketing studies to answer questions about long-term toxicity and benefits. Adaptive licensing will be key to allowing high-quality real-life prospective cohort studies to gather data on efficacy and safety data throughout recurrences, which can provide benchmarks to guide future decisions on access. EORTC, which has historically put an emphasis on following patients who participated in their studies, is now entering partnerships with European cancer registries to find ways of better exploiting the vast resources of data available on how treatments work over time and in real life.
So the days of talking about phase I, II and III trials in cancer drugs are numbered, says Lacombe. “I like to speak instead about clinical trials that are designed to understand and clinical trials that are designed to conclude – we need to change the terminology to change the mindset, because a number of people out there are still thinking about phase I trials in the classical sense, where they were designed to test safety. If we change the mindset, we can change what we achieve.”
“The diabolo shape of product development is what the patients want, what the drug regulators want, what the payers want. It’s really answering questions for the subset of patients that actually benefit, so that you avoid undue toxicity for those who don’t benefit. And the payers, in any case, aren’t going to be able to support treatments for small numbers of patients unless we come up with this evidence of clear benefit.” “As for patients, it’s like when you buy a car: if you put the key in, you expect it to start. People on trials expect the drug to function for them – but that doesn’t work with the classical trial model.”
“People on trials expect the drug to function for them, but that doesn’t work with the classical trial model.”
This is why EORTC has completely repositioned itself over the past decade, says Lacombe, who presented the diabolo concept two years ago in a paper with EORTC colleagues published in the European Journal of Cancer. Today there is far more emphasis on trials examining biology, developing biomarkers, and bringing together a range of health and scientific disciplines to work with patients in translational research. “Two things have always characterised EORTC trials,” says Lacombe. “They are international and multidisciplinary. So for us, it is an entirely logical evolution.”
EORTC was founded on those principles in 1962, building a scientific and operational infrastructure for investigator-driven clinical trials and translational research. It is independently funded through various sources, including national cancer leagues, although some drug studies are conducted in cooperation with pharmaceutical industry partners.
Lacombe gained an early interest in research when he simultaneously trained in medicine and pharmacology in Marseilles in the 1980s. He first arrived at EORTC in 1993, having spent the previous two years working for a small French drug company, and three years before that on a post-doctoral fellowship at the Roswell Park Cancer Institute in the United States. Whereas his time in the United States, where he researched chemotherapy in advanced breast cancer, had opened his eyes to the wide potential of research – “Being in America changed my life” – research within industry felt restrictive and inflexible in comparison. And when his company conducted some research with EORTC, Lacombe was fascinated by the excellence of the European organisation’s trial design.
He contacted Françoise Meunier, EORTC Director General from 1991 to 2015, who offered him a fellowship, because she needed someone with experience of industry and pharmacovigilance.
He took up the offer, wanting a new challenge, and has stayed with EORTC ever since, becoming medical supervisor in 1994, and then moving on to head the investigational agent unit, the intergroup office, and the pharmacovigilance and regulatory affairs units (both of which he set up), becoming Assistant Director of Medical Affairs and New Drug Development in 1998, Scientific Director in 2007 and Director of EORTC headquarters in 2010. Today, alongside his strategic role, he has daily responsibility for running the headquarters and managing its staff.
Lacombe may still be shocked that he leads EORTC after all these years, but there can be few who know the organisation or its research environment as thoroughly.
“In the early ’90s, the organisation was data managers and statisticians, so I brought my knowledge about how to build the infrastructure you need beyond clinical trials – and it was very important, because by the late ’90s, the regulatory frameworks started to change.” When Lacombe arrived, EORTC employed 30 people. Today it employs 175.
For all the change, Lacombe insists that the mission remains the same, with the organisation still valuing its long-term capacity to follow patients and update old trials, while repositioning itself to revolve around new trials that understand the mechanism and biology of disease rather than randomising patients to test a hypothesis.
Fundamental to the shift has been addressing a basic question: if new targeted drug development depends on sorting patients according to their molecular features, how do you optimise access to the right kind of patients for trials? And how do you make sure that patients have access to the maximum number of trials that are likely to benefit them?
EORTC’s answer has been to establish a molecular screening platform called SPECTA (Screening Patients for Efficient Clinical Trials Access). It depends on academics, clinicians and industry working with EORTC to share and contribute to a biobank and database of patient molecular profiles.
“With our access to large territories and a large number of patients, we can set up platforms where patients are molecularly defined and sorted. This is a knowledge development platform, a clinical trial access programme, and it also increases the likelihood of being able to offer to patients the best treatment known so far.”
“The concept is to first understand the biology, and then propose a clinical trial, not the other way around.”
The first SPECTA platform, in colorectal cancer, started in 2013, and 700 patients in trials across Europe have so far agreed to join the programme. The lung cancer platform opened in June. It is early days, but Lacombe is passionate about SPECTA’s significance – and not just because of its practical applications. It may help bring the demise of current research systems which result in a tragic waste of biological data and material.
“Currently, three companies might screen 2,000 patients for the 200 they need for their trial. They store the material of the 2000, but ultimately there are 1,800 they don’t care about because they are not the target. For 5,400 people the material is locked away for no purpose. What we’re proposing is that instead of the companies screening 6,000 patients, we screen 2,000 and can drive 600 patients to three different kinds of studies.”
“I don’t understand it when ethics committees approve studies where biological material goes into a commercial silo – it is too scarce to be lost.” Lacombe calls such biobanks “butterfly collections”: an array of beautiful things left without use, gathering dust.
“I don’t understand it when ethics committees approve studies where biological material goes into a commercial silo”
“It’s not the fault of pharma – they must have access. But, as a community – pharma, academia, the patient, the regulator – we must be able to find a better way to access and share in a collegial multidisciplinary way. The patients are telling us that is what they want: they are saying ‘Use, re-use, abuse our material, bring knowledge.”
It’s a fine vision. But I put to him the historic problems of widespread collaboration and sharing – not just because of industry’s defence of intellectual property, but a possessiveness of knowledge in academia.
Lacombe points out that a wide range of stakeholders – the European Medicines Agency, industry, government, regulators, payers – have been involved in consultation and planning for the EORTC platform.
“Sharing is certainly a challenge, but it’s part of the change of mindset that we need in the new environment, and I want to believe that these programmes will help. Things are now too complex and too expensive to do by yourself.” But how does EORTC manage to sell the added value of collaboration to all these different parties, when tradition, scientific egos – even funding – keep everyone in professional and institutional silos?
Lacombe admits the honest answer is that he does not know. “The only thing I can tell you is that we now have a waiting list of institutions who want to join these programmes. I think you can imagine that Europe is a challenging environment, because there are multiple companies, multiple regulations. But we have so much capacity – and have achieved much more with much less than the United States, and I want to believe that this part of the world is much more innovative. Maybe I’m too naive, a dreamer.”
“It’s complex, expensive, difficult, but the early signs are good. I believe that things in the future aren’t going to be academia, industry, regulator separately. Everything will be much more integrated.”
Lacombe’s optimism is partly founded on his belief that the partnerships that EORTC has been forging over the past decade and more will prime a more general process of sharing and collaboration. He refers to partnership 40 times during our two-hour conversation, citing EORTC’s partnerships with: the European Society of Pathology, to help establish biobanks and their quality assurance processes; the European Society of Radiology and other imaging societies to help it establish imaging platforms; the European Thoracic Oncology Platform on lung cancer research and establishing SPECTA lung; the European Society of Surgical Oncology on a surgical quality assurance programme. He is keen to emphasise how innovative the latter partnership is, building on EORTC’s multidisciplinary agenda to place surgery at the centre of research.
His point is that research needs are so complex now that EORTC cannot do it alone – and neither can anyone else.
“So complex are research needs now that EORTC cannot do it alone – and neither can anyone else”
“If there is one thing EORTC knows about, it’s infrastructure for international multidisciplinary clinical trials. If we can partner with those who have another area of expertise, we can define new questions and make it happen together.”
“So yes, maybe individual pathologists might say: ‘Why should I send my biological material to EORTC?’ But if you are a partner with the Society of Pathology, then you create a certain dynamism around the whole project.”
Lacombe believes in Europe’s potential for collaboration, despite the EORTC having faced several specifically European problems over the past ten years. Economic pressures on the pharmaceutical industry in Europe affected how the EORTC collaborated with them: “We had to adapt and stipulate that we only wanted to conduct good studies with a good amount of support from them.”
And then there have been the problems posed by new regulations on clinical trials, medical devices and data protection, due to be introduced in EU countries in 2016. EORTC has been active in voicing the concerns of academic research. Lacombe believes that one of the major challenges the cancer research community will now face is how to implement the data protection regulations without damaging clinical research. Roger Stupp, EORTC’s President, has been vocal in pointing out that time-consuming paperwork is already stifling innovation in research and the ability to share vital data.
“The problem is that people who do regulation sometimes don’t understand,” says Lacombe. “They want a single regulation for data protection, but my banking data is a completely different thing than my biological data. That’s a big problem.”
Regulators also initially failed to understand that more than 50% of clinical research in the field of cancer was not in drug development – with many standards of care based around combinations of drugs, radiotherapy and surgery.
Nevertheless, the new regulations have been improved with the input of EORTC and academic partners. “I think we’ve helped push forward the simplification of procedures, and helped define low-risk clinical trials – those that are performed without drugs, or are about optimising treatment rather than new treatments – where procedures may need to be less rigorous.”
So, he feels the challenges posed by the new regulations are not insurmountable: “We were all concerned 10 years ago when the clinical trials directive came in, but we survived it. I think we will pass this challenge too. It’s just a pity that we have to use our energies on this when they would be better placed elsewhere.” Equally, the difficult economic climate, and the expense of traditional means of drug development, will force change in industry and other stakeholders, says Lacombe.
“It’s a little bit unfortunate that it has to happen this way, but it’s possible that because of the economic pressures we will force people to think differently. I think we should all be anticipating change so that we have capacity to do new kinds of research, but soon, instead of anticipating, people will just be faced with this new situation. Some drug companies are already facing it, and that’s why we are getting a lot of enquiries about our SPECTA programme: Can you help with long-term follow-up? Can you help with benchmarking? Can you help us access this population?”
“All stakeholders need to find new ways to interact. Maybe industry takes more time – I understand they have pressures and shareholders to consider – but we all have to accept that we now have to leave our comfort zone. This is not yet happening, so we are doing a lot of communication to try and make it happen.”
Lacombe might be the right person to bring this off. He is not a pushy performer – he acknowledges he is shy and is genuinely flattered by the ‘personal recognition’ that a Cancer World interview brings. But he has an infectious enthusiasm about the potential for European cancer research and everything to do with EORTC. This isn’t just a job for him.
“Basically, I do only three things in my life because I have no time for anything else: my work, my family and my jogging. That’s what I do.”
But for all his natural quietness, Lacombe is confident he’s the right person for the job. “I think there is a natural selection process of people who have energy and passion,” he tells me as we conclude the interview. He knows he may be accused of being a dreamer, but he also knows that following a vision for research in Europe is the only way it can now move forward.
It’s a message he has passed on to his children, now aged 12 and 14. “I always say to them, you need a passion to start, and then a vision to continue. You need to want it.”






Leave a Reply