Once used primarily to detect large polyps and tumours, endoscopy has now become an essential tool for early diagnosis, curative treatment and palliation for gastrointestinal cancer.
This is an edited version of a presentation delivered by Michael Häfner, from St. Elisabeth Hospital, Vienna, Austria, as a live webcast for the European School of Oncology. It is edited by Susan Mayor. The webcast of this and other e-sessions can be accessed at e-eso.net.
Endoscopy is used in gastro-intestinal (GI) oncology in diagnostic, curative and palliative settings. In diagnosis, it is used to detect lesions, facilitate macroscopic classification of tumours and enable biopsy and fine-needle aspiration to collect tissue samples. In curative treatment it is used to resect early cancer by endoscopic mucosal or submucosal resection and thermal ablation. In palliation it is used mainly for stent implantation, but also for percutaneous endoscopic gastrostomy.
Endoscopy in diagnosis
In the early days endoscopy was used mostly for diagnostic procedures with the goal of finding tumours – mostly large polyps and tumours at an advanced stage. The development of more advanced technologies over recent years has led to a paradigm shift, and endoscopy has moved on to the detection of small, flat, early malignant or precancerous lesions. The benefit is that there are now several interventions to cure these conditions, including resecting early lesions by endoscopic techniques.
Technological developments, inclu-ding high-resolution endoscopy and zoom endoscopy, enable clinicians to examine lesions more closely, for example assessing the vascular pattern in dysplasia in Barrett’s oesophagus. Novel technologies, such as confocal laser endomicroscopy and autofluorescence, now allow the detection and characterisation of minute lesions that can otherwise be difficult to see. This is crucial because mucosal cancer can be cured by means of endoscopic resection in many cases.
The figure overleaf shows an example of real-time pathology using confocal endomicroscopy, which is typically carried out using a probe during endoscopy, such as during endoscopic retrograde cholangio-pancreatography (ERCP) in the bile duct. In this case the procedure was carried out during gastroscopy with a dedicated endomicroscopy endoscope in a patient with Barrett’s oesophagus. The image shows a leakage in the bright area, which is the contrast agent given intravenously, and a distortion of the mucosal cells that indicates Barrett’s cancer. The benefit of this technique is that it offers real-time histopathology while examining a patient, without taking a biopsy. Based on these findings, we were able to plan our therapeutic intervention. Only a few centres in the world currently have this technology, but it is an exciting development for the future.
To actively find lesions it must be clear what we are looking for. Classifications are usually considered rather boring, but they can be very useful in endoscopy. The figure below shows the Paris endoscopic classification for superficial neoplasia, which applies in all of the organs that can be reached with an endoscope: the oesophagus, stomach and colon.
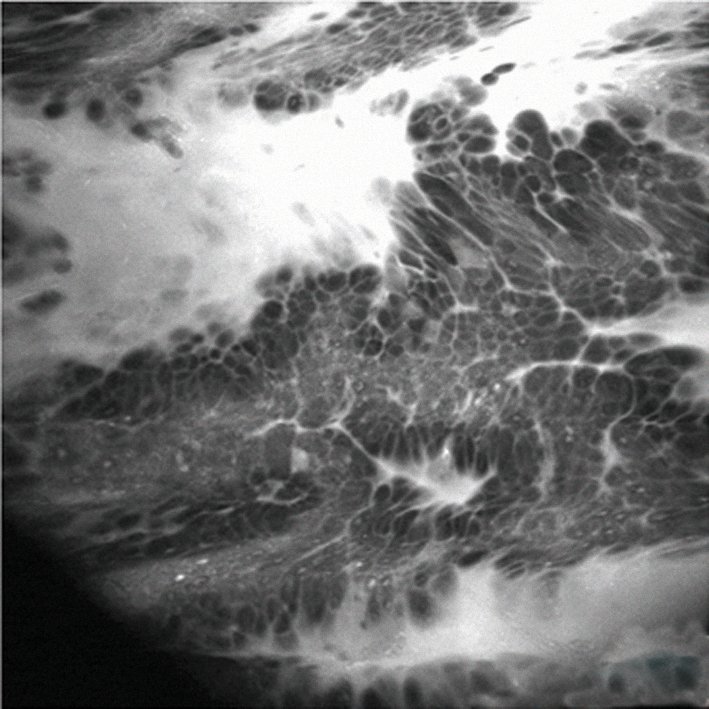
Confocal endomicroscopy reveals leakage of contrast agent (bright area) and a distortion of the mucosal cells – enough to diagnose a Barrett’s cancer without the need for biopsy
Courtesy of Michael Häfner
Enhancing images
There are several techniques for enhancing endoscopic images. Flat lesions can be very difficult to detect, so considerable knowledge of macroscopic features is needed. The endoscopic picture can be enhanced using optical or electronic manipulation, with techniques such as virtual chromoendoscopy, or use of a dye in chromoendoscopy. The goal is to enhance contrast and improve the detection of lesions and allow for their characterisation, including assessment of surface patterns. Virtual chromoendoscopy makes use of the fact that modern endoscopes are basically computers that allow manipulation of colours, essentially providing ‘Photoshop’ for endoscopy. Other techniques use optical filters that enhance the contrast and make blood vessels and surface patterns more visible, enabling us to spot very discrete lesions.
The figure on page 39 (top left) shows a completely flat lesion on virtual chromoendoscopy, which makes lesions easier to see by changing their colour. This was a squamous cell cancer of the oesophagus that was extremely important to diagnose, because endoscopic sub-mucosal resection cured this early cancer with no need for surgery. Lugol’s staining shows the lesions much more clearly as the unstained areas. The case illustrates that it is important to know what you are looking for, or you may miss subtle lesions.
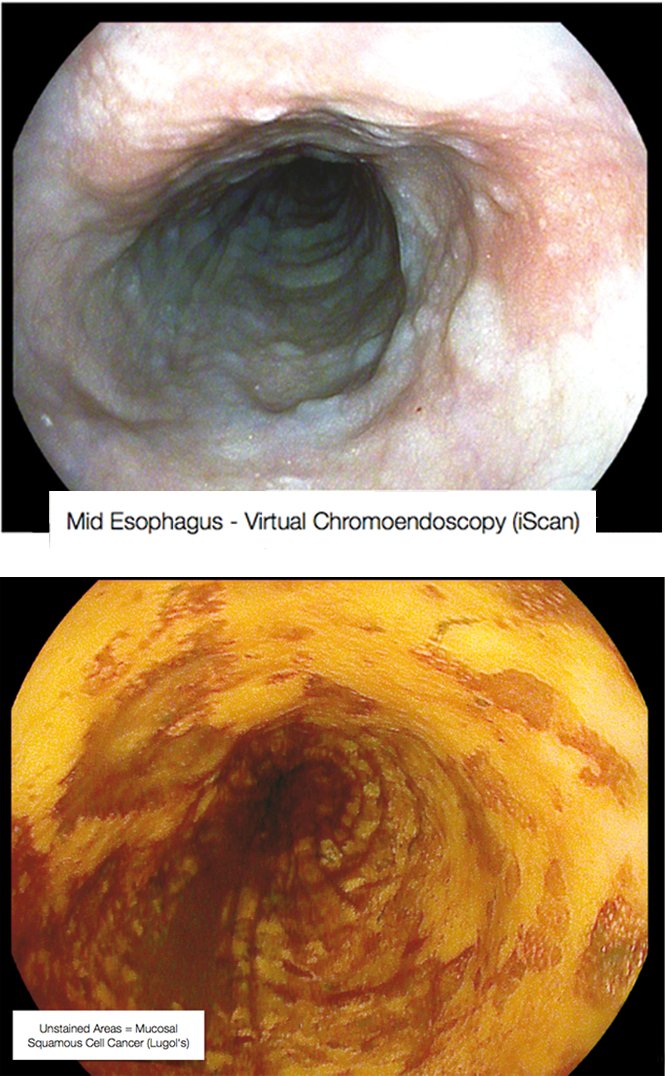
Mid-oesophagus scan using virtual chromoendoscopy (above) and Lugol’s staining (below) in the same patient – Courtesy of Michael Häfner
If these technologies are not available, various dyes can be used to improve image visualisation throughout the gastrointestinal tract. This can facilitate detection and also characterisation of a lesion. They include:
o Lugol’s staining. This is iodine based (so it is important to be aware of the risk of allergy), and is used for the detection of oesophageal squamous cell cancer. It is also used for screening high-risk patients, such as smokers or patients with a history of alcohol abuse.
o Acetic acid (vinegar). This reacts with the surface of the mucosa and makes the surface pattern, or pit pattern, of a lesion much more visible. It is applied to detect dysplasia in Barrett’s oesophagus.
o Indigo carmine. This can be considered the ‘Swiss Army knife’ of gastrointestinal endoscopy, and is widely used throughout the gastrointestinal tract. The blue colour stains depressed areas and increases the contrast. It also makes surface structures, surface patterns and the margins of a lesion more visible (see page 39 top right). The spread and surface pattern information can help distinguish benign from malignant lesions.
Modern imaging technology makes it possible to reliably predict the histopathology of lesions. Macroscopic classification, with the help of contrast enhancement, is important for making decisions during endoscopy on whether to resect a lesion, take a biopsy, refer for surgery or leave a lesion because it has no risk of malignancy. Commonly, classifications are used for (early) oesophageal cancer, (early) gastric cancer and lesions in the colon. The oldest and most widely used classification, Kudo’s Pit Pattern (see figure right), has been developed for colon polyps. Certain types of patterns are clearly associated with neoplastic lesions and others with non-neoplastic lesions, providing further information for decision-making during endoscopy without having to wait for pathology results.
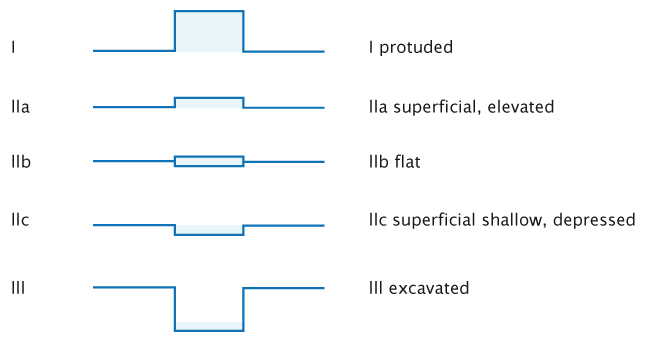
The Paris endoscopic classification of superficial neoplastic lesions. Source: Participants in the Paris workshop (2003) Gastrointest Endosc 58:S3–S43 Reprinted with permission from Elsevier
Fine-needle aspiration
Not everything is accessible for direct biopsy. Tissue sampling from deeper layers, for example submucosal lesions, is usually unsuccessful with standard biopsies. Some lesions cannot be reached at all because of their nature or location, such as lesions in the lymph nodes or outside the gastrointestinal tract, such as the pancreas. In these situations, endoscopy-guided fine-needle aspiration is a relatively easy way to obtain tissue samples. It is ultrasonography based and enables operators to puncture tissue and obtain pathology specimens. However, results can vary considerably depending on operator experience, the presence of a cytologist, who can advise when enough material has been obtained, and the choice of needle.
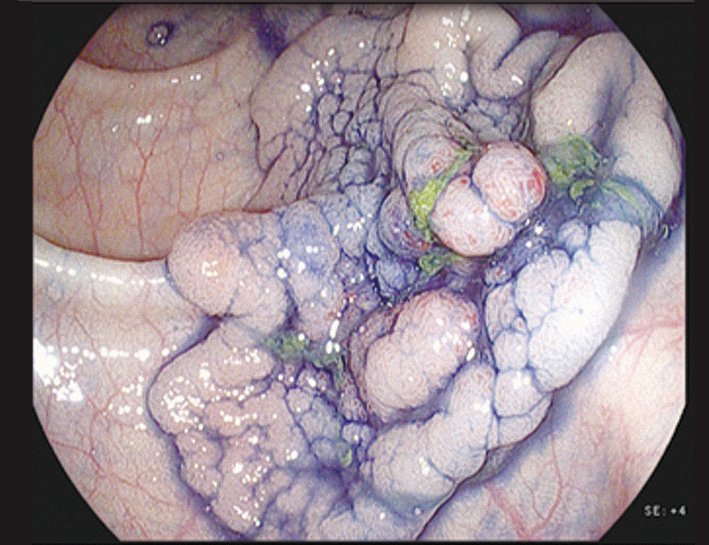
Staining with indigo carmine shows up the margins of the lesion and its surface patterns well enough to reliably predict the histopathology – in this case a later- ally spreading tumour with granular type pit pattern type IV
Courtesy of Michael Häfner
Question: Would you consider taking a biopsy in early malignant lesions?
Answer: It is important to recognise early lesions because taking a biopsy is not generally recommended due to the risk of making resection more difficult. It is best to resect flat lesions immediately or mark for a colleague who will resect them. You can biopsy any polypoid lesion safely without having consequences for endoscopic resection.
Endoscopy in curative therapy
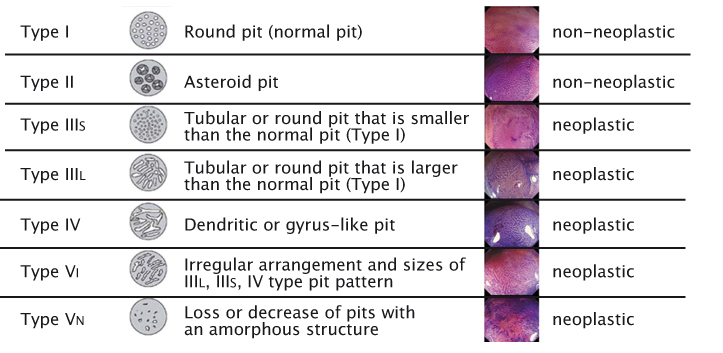
Source: S Kudo et al. (1996) Gastrointest Endosc 44:8–14 Reprinted with permission from Elsevier
Use of endoscopy in curative treatment of gastrointestinal oncology is a very recent development. In the past, the standard treatment of gastric cancer was surgical, but endoscopic techniques have been developed so that endoscopic mucosal resection (EMR) of early cancers is now the standard approach. The first attempt at endoscopic treatment was reported in Japan in 1974, where it was used to treat a polypoid-type gastric cancer. Subsequently, EMR techniques have been developed to resect flat gastric lesions. You can usually resect lesions of up to 1.5–2 cm in one piece. However, this technique is unsuitable for larger lesions, which have to be resected piecemeal or with a newer technique called endoscopic submucosal dissection (ESD). This has, theoretically, no upper limit on the size of the lesion that can be resected.
Endoscopic mucosal resection was developed initially for the treatment of mucosal gastric cancer in Japan. It is currently used for lesions in the oesophagus, where it is used in Barrett’s oesophagus, in the stomach and the colorectum. The main drawback of this method is the limited size of specimens that can be resected en bloc; but, on the positive side, it is fairly quick to learn and is safe. A commonly used technique is cap-EMR, in which saline, and sometimes dye, is injected into the submucosa. The artificial polyp created is sucked into the endoscope cap and cut with a snare. Complications are rare and include bleeding (5%), perforation (very rare) and strictures, which are also rare but may occur, for example, in circumferential EMR in the oesophagus. The figure top left shows a Barrett’s cancer pre- and post-EMR.
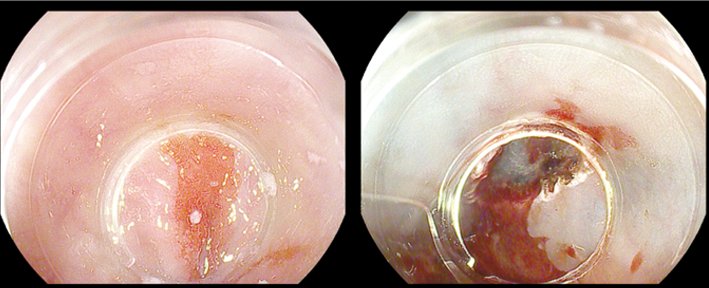
Courtesy of Michael Häfner
Endoscopic submucosal resection overcomes this limitation, allowing for en bloc resection of larger lesions if certain criteria are met. It is now considered the treatment of choice for intramucosal gastric cancer and oesophageal cancer, especially for squamous cell cancers, and is superior to conventional EMR in terms of the curative and recurrence rates.
Endoscopic submucosal dissection (ESD) is a new development in therapeutic endoscopy, which allows the direct dissection of the submucosa and enables large lesions to be resected en bloc. ESD is not limited by resection size and is expected to replace surgical resection, at least in well-defined indications (J Gastroenterol 2006, 41:929–942). However, it is associated with a higher incidence of complications than standard EMR procedures, is much more complicated to perform than EMR and requires a high level of endoscopic skill.
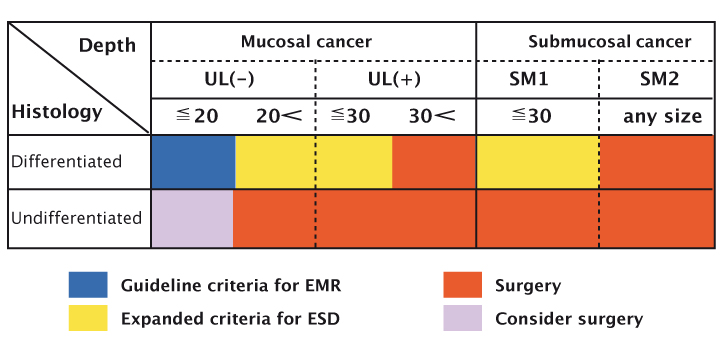
Source: T Gotoda, H Yamamoto and RM Soetikno (2006) J Gastroenterol 41:929–942 Reprinted with permission of Springer
Indications for treatment
The most important indications for endoscopic treatment of early gastric cancer are determined by considering the risk of lymph node metastasis, technical problems and whether to resect the tumour en bloc. The conventional criteria for endoscopic resection of early gastric cancers, which were proposed by the Japanese group, are:
- highly differentiated adenocarcinoma
- intramucosal cancer
- size of the lesion less than 20 mm
- no endoscopic findings of ulceration
- no lymph node involvement or metastasis seen on computed tomography.
Lesions that meet all these criteria should be considered for en bloc resection by conventional EMR methods because of the low risk of lymph node metastasis.
Extended indications for EMR have recently been proposed, based on surgical data. Gotoda et al. reported that lesions meeting the following extended criteria have no, or minimal, risk of lymph node metastasis (J Gastroenterol 2006, 41:929–942):
- no size limitation for intramucosal differentiated cancers without ulcer-a-tion that have no lymphovascular invasion
- less than 3 cm in diameter for ulcerated differentiated intra-mucosal cancers without lympho-vascular invasion
- less than 3 cm in diameter for differentiated cancers (extension into the submucosa <500 µm) without lymphovascular invasion
- less than 2 cm in diameter for undifferentiated intramucosal cancers without ulceration.
EMR can be performed, sometimes in an outpatient setting, in patients meeting these criteria, and it provides a very effective way of treating patients. The figure at the base of page 40 presents a graphical representation of the criteria, illustrating that EMR and ESD can be used for small lesions, nonulcerated lesions, and lesions limited to the mucosa. Patients with other types of lesions should be considered for surgery.
Endoscopic submucosal dissection technique
Endoscopic submucosal dissection (ESD) involves applying a submucosal injection, which can be with saline or glycerol, although we prefer to use hyaluronic acid for more complicated procedures because the cushion it forms in the submucosal layer is longer lasting. ESD knives are available in different shapes, including the IT knife, which is designed to reduce the risk of perforation. Haemostasis devices, such as the coagrasper that coagulates blood vessels in the submucosa, are required because bleeding is common, as for EMR. In addition, devices are needed for closing perforations. These may include conventional clips or OTS clips, which can close larger perforations.
A 2011 study of ESD from the French working group of 16 centres, each with one endoscopist performing ESD, analysed data from 188 consecutive patients (mean 6 per centre, range 1–43) (Endoscopy 2011, 43: 664-670).
The cancers treated with ESD were: stomach (n=75), oesophagus (n=27), duodenum (n=1), caecum (n=3), right-sided colon and transverse colon (n=8), sigmoid (n=3), and rectum (n=72). The mean size of lesions treated was 26 mm, with the largest being 150 mm. Most lesions were high-grade dysplasia or mucosal cancer (71.2%). En bloc resection was performed in 77.1% of cases, which was a little bit worse than figures from Japan, and the R0 resection rate was 72.9%. The mean duration was 105 minutes and the complication rate was 29.2% (34 perforations, which is high, and 21 cases of bleeding), with most resolved conservatively.
The number of ESD procedures being carried out is increasing, and their duration and complication rates are decreasing with growing experience. In my unit we performed 50 ESD and EMR procedures in 2014.
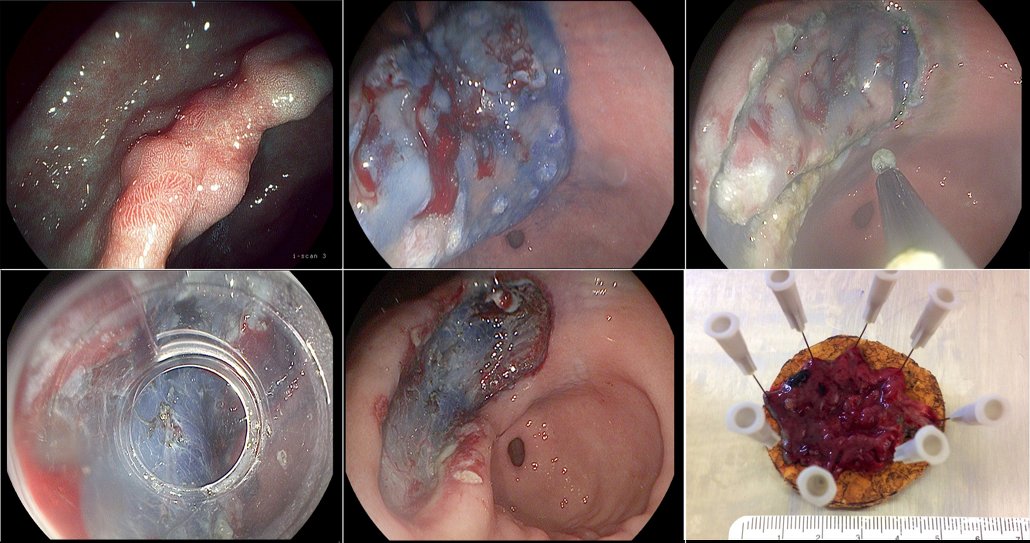
Endoscopic submucosal dissection of a gastric cancer
Figure a) shows a clearly suspicious stage IIa–c lesion in the stomach, which had been biopsied in the referring hospital and found to be a gastric cancer. We marked the outer margins and administered a submucosal injection of hyaluronic acid (b), creating a cushion that gave enough space to enable us to resect the lesion in the submucosal layer and in one piece. The next step was to cut around the lesions (c), with indigo carmine used to stain the lesion margins, before mounting the cap (d) and continuing to resect in the submucosal layer and remove the lesion (e). Pathology showed the tumour was limited to the lower third of the mucosal layer, was highly differentiated and there was no lymph node involvement (f). The patient was cured of her gastric cancer by this endoscopic dissection procedure.
Palliation
The goal of endoscopic palliation is to offer minimally invasive therapy to reduce a patient’s suffering and to avoid surgical interventions. Common procedures include bile duct drainage, implantation of endoprostheses in other areas of the gastrointestinal tract, and endoscopic feeding tubes (percutaneous endoscopic gastrostomy, PEG). Although endoscopic palliation is commonly performed, some indications, such as colonic stenting, have to be chosen carefully.
Stenting
Stenting is a very important method for palliation in endoscopy. Stents are most commonly used in the bile duct as a palliative measure in unresectable tumours, such as tumours of the pancreas or cholangio-carcinoma. Plastic stents are cheap and widely available but suffer from limited patency, for example biliary stents last for about three months. Metal stents are expensive, but have much longer patency. However, in the case of covered stents they can’t be removed. Other common indications for stenting include oesophageal cancer, duodenal obstruction due to pancreatic cancer and, in rare cases, colon cancer.
Percutaneous endoscopic gastrostomy (PEG)
Common oncological indications for endo-scopically placed feeding tubes include:
- oro-pharyngeal and oesophageal malignancy with inability to eat
- prolonged stomatitis after radiation therapy for oropharyngeal cancer
- oesophageal fistula and perforation.
A PEG should be considered if prolonged enteral feeding is required for a period of longer than three weeks. PEG placement is generally considered to be relatively safe. Complications such as infection occur quite frequently, but can be avoided by giving a single injection of an antibiotic.
Question: In times of limited resources, which patients are best for endoscopic palliative treatment?
Answer: As a rule of thumb, I would suggest patients who are fit enough to go home after palliative interventions are good candidates. Conservative methods may be preferred for patients in their final days, because of the risk of complications and the limited potential for gains in quality of life and survival time. We discuss options with the patient and their relatives and come to a decision together.
Take home messages
“Seeing in endoscopy” has changed considerably over the last few years. Detection of premalignant lesions or early cancer has become the pinnacle of diagnostic endoscopy.
High- and low-tech image manipulation allows for precise characterisation of a lesion based on its macroscopic appearance.
Endoscopic resection techniques such as submucosal dissection can replace surgery in carefully selected patients.
Endoscopic palliation should be considered as a minimally invasive option in patients with advanced oncologic diseases.



Leave a Reply