This egrandround was first presented by Caroline Moore, from University College Hospital London, as a live webcast for the European School of Oncology. It is edited by Susan Mayor. The webcast of this and other e-grandrounds can be accessed at e-eso.net
Until the last few years MRI was used essentially as a staging tool in prostate cancer, with imaging being performed after a biopsy to assess a patient’s suitability for radical treatment and for assessing extraprostatic extension and disease. However, it has much greater potential, and is now being used at several different stages in the prostate cancer pathway.
First, and I think most importantly, MRI can be used to detect and localise prostate cancer. As in the past, it can be used to stage prostate cancer, but it can also be used to plan and guide treatment very specifically, to assess the completeness of treatment and to monitor for recurrence. MRI can also be used for surveillance in men with prostate cancer where we consider their disease is not significant enough for treatment and we want to detect change in cancer volume or grade.
Using MRI to help decide who to biopsy
In a systematic review looking at studies comparing MRI-targeted prostate biopsy with standard transrectal biopsy (European Urology 2013; 63:125–140) we found MRI-targeted biopsy achieved more efficient sampling, with equal detection of clinically significant disease but with fewer cores, and with fewer men needing biopsy. There was also less clinically insignificant disease detected and more effective assessment of the extent of disease, including cancer core length, representing tumour burden, and Gleason score.
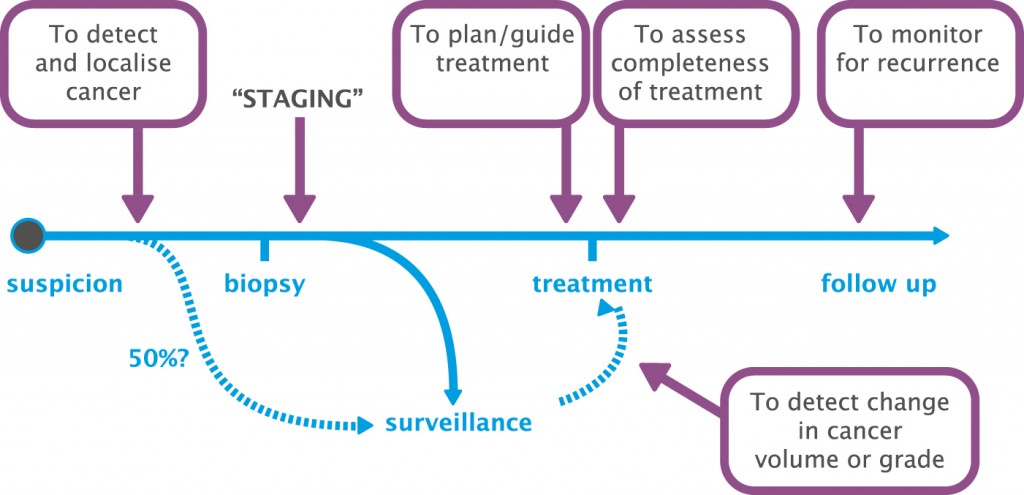
Carrying out an MRI-targeted biopsy alone would miss some men: 51 of 555 men (9%) with a negative MRI had cancer on standard biopsy. However, significant cancer (>4 mm cancer core length, any pattern 4) would be missed in only 2.3% (13/555) of men referred for a biopsy. On the positive side, one in three men would avoid a biopsy, and the troublesome problem of an insignificant cancer being diagnosed would be avoided in one in 10 men.
Reporting of groups of men who have MRI-targeted and standard biopsies is not always sufficient for us to compare the two different approaches, so we convened an international working group to look at this issue. The group recommended:
- Standard and MRI-targeted cores should be reported separately using separate Gleason scores and maximum cancer core lengths for both.
- A comparison table of clinically significant disease using each approach should be given in studies comparing the two types of biopsy, giving the numbers of patients with no cancer, with clinically insignificant disease, and with clinically significant disease for each biopsy strategy.
- A new definition of clinical significance will be needed for MRI-targeted biopsy studies.
Since these recommendations were made (European Urology 2013; 64:544–552), a large study carried out at the National Institutes of Health comparing standard transrectal biopsy with MRI-/ultrasound-guided biopsy in 1003 men included the suggested comparison table (JAMA 2015, 313:390–397), which demonstrated that the likelihood of missing an important cancer is much higher with a standard biopsy than with an MRI-/ultrasound-guided biopsy.
Using MRI to help decide how to biopsy
MRI information can enable a biopsy to be carried out much more accurately, which means it can be approached in a different way to the standard template of the transrectal biopsy, where you might decide the number of cores based on the volume of the prostate, but stick to a standard template for how you take those cores.
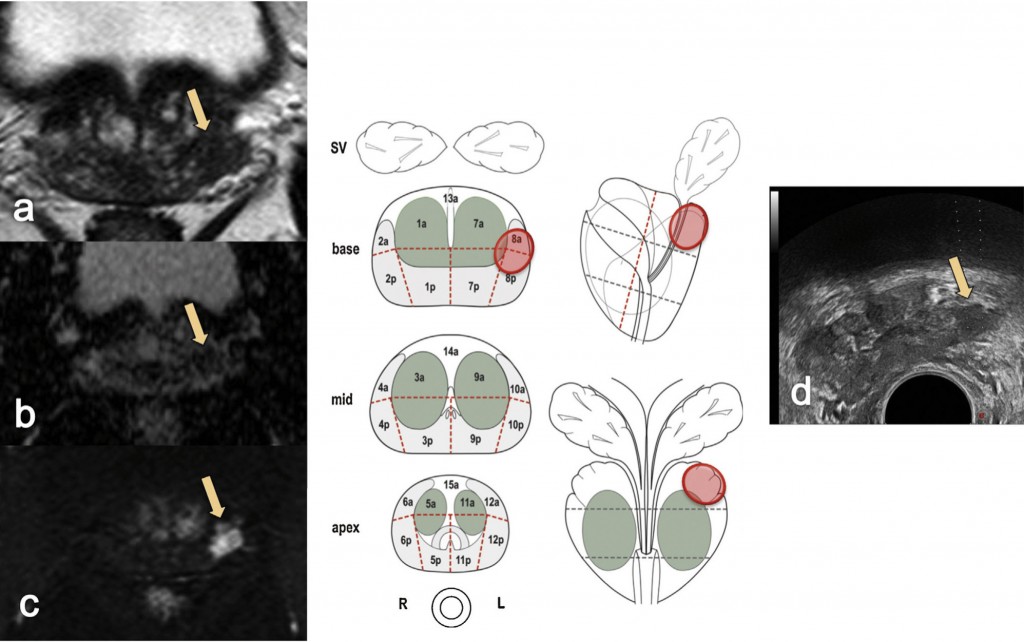
The most common way of carrying out an MRI-targeted biopsy to sample a lesion seen on MRI is by using visual registration (see figure opposite). The radiologist reports the MRI scan, ideally in a diagrammatic form, drawing the lesion in different sections on the prostate or by annotating the MRI images.
A number of groups, including ours, are using software registration that transposes MRI information onto the ultrasound information used at the time of biopsy. This gives the advantages of an ultrasound clinic-based approach at the same time as allowing the important MRI information to be transferred.
Head-to-head studies of visual registration and software registration suggest there is no clear winner at the moment. It seems to depend on both the tumour – larger tumours being more easily accessed with visual registration – and on the expertise and experience of the radiologist reporting the scans and the operator performing the biopsies.
The third method, less commonly used, is MRI-targeted biopsy using an ‘in-bore’ biopsy device that allows you to see the needle in the MR machine. Most centres perform a high-resolution diagnostic scan first and then an interventional scan at the time of the biopsy. The diagnostic MR images and the interventional images would be co-registered.
Question and answer
Question
If you have a patient who does not have an MRI before biopsy, how long would you wait to perform MRI after biopsy?
Answer
For me, it depends on the cancer you find at the biopsy. If you find, for example, 10 mm of Gleason 4+3 high-risk disease and you just want to know about the nodes or extraprostatic extension, I would do that fairly quickly because the nodes will not be affected by biopsy. But if you find 2 mm of 3+3 disease and you think that a man is suitable for surveillance based on the standard biopsy, then I would tend to wait at least 3 months before doing an MRI scan. We know that for some men, even at 3 months, there will still be changes that show up on the scan and make it more difficult to interpret.
Question
UCL have been pioneers in dissemination of template biopsies. Is there still a role for template biopsies in the era of MRI?
Answer
It depends what you mean by template biopsy. A 5 mm mapping biopsy taken in a detailed and intensive manner should not be necessary in the future, based on data that we have been collecting. It may be necessary in some cases, such as those men who are not suitable to have an MRI scan, those with a worrying PSA where no cancer is evident on MRI or standard biopsy. Our usual approach at UCL is to carry out targeted biopsies and then, for men undergoing a primary biopsy, we might do a less intensive 12-zone transperineal biopsy rather than a full 5 mm mapping biopsy.
MRI in active surveillance
A systematic review of the literature (European Urology 2015; 67:627–636) reveals three different datasets of interest for MRI in men on active surveillance.
Radical prostatectomy data
In men who are suitable for surveillance on biopsy criteria, what sort of disease is found if you go ahead with a radical prostatectomy? The review found for men with a positive MRI there was a 44% chance of upgrading from being suitable for surveillance to being unsuitable. There was a much lower chance (11%) for men with a negative MRI. There was less of a difference for upstaging based on MRI status as opposed to biopsy status: 25% of men with a positive MRI were upstaged and 10% of those with a negative MRI.
Reclassification biopsies
If a man has a biopsy suggesting he is suitable for active surveillance and he has a concordant MRI (a positive MRI with a small lesion or a negative MRI with no specific lesion), then the likelihood of reclassification on repeat biopsy is 17%. If, however, there is a discordant MRI (the MRI suggests more significant disease) then there is a 77% likelihood of reclassification, which is quite a strong driver to perform extra biopsies if the MRI does not match a patient’s current biopsy.
Repeat MRI on surveillance
 I am particularly interested in this area, which involves looking to see if we can use MRI for active surveillance instead of repeat biopsy. It is essentially looking at radiological progression, which means an increase in volume or in the conspicuity of a lesion on MRI. The data showed that men with a positive MRI at baseline had a one in three (32%) chance of radiological progression over a three-year period. Men with a negative MRI at baseline had an 11% chance of radiological progression over this period.
I am particularly interested in this area, which involves looking to see if we can use MRI for active surveillance instead of repeat biopsy. It is essentially looking at radiological progression, which means an increase in volume or in the conspicuity of a lesion on MRI. The data showed that men with a positive MRI at baseline had a one in three (32%) chance of radiological progression over a three-year period. Men with a negative MRI at baseline had an 11% chance of radiological progression over this period.
There is a lot of work to be done to define these elements of progression, but I think this is an interesting area for the future. The challenges are in how we define radiological significance and then how we define progression. The RECIST criteria that we use in a lot of other tumour groups would need a minimum of a 1 cm-diameter tumour, which would be uncommon in a patient put on surveillance for localised prostate cancer. We also need to look at standardised reporting for volume, change in volume, and change in characteristics. We don’t yet know how important it is if a lesion becomes visible on diffusion imaging when it was not previously visible, although we suspect it means higher-grade disease and that we should probably be taking action. Once we have answered all these questions, the final challenge is what to do with the information for the care of the patient in front of us.
Question and answer
Question
Is there a minimal volume that MRI can detect or is it always according to grade?
Answer
It is grade-dependent. MRI has excellent ability to detect any tumour of 0.5 ml or more when it is a focal lesion. It is still very good in detecting tumours of 0.2 ml and less, particularly if they are high grade, for example tumours of 0.1 ml with primary Gleason pattern 4 element will show up quite well on MRI. This works well for focal lesions, but with diffuse change throughout the peripheral zone you can have a reasonable volume of a low-grade tumour that does not show up on MRI.
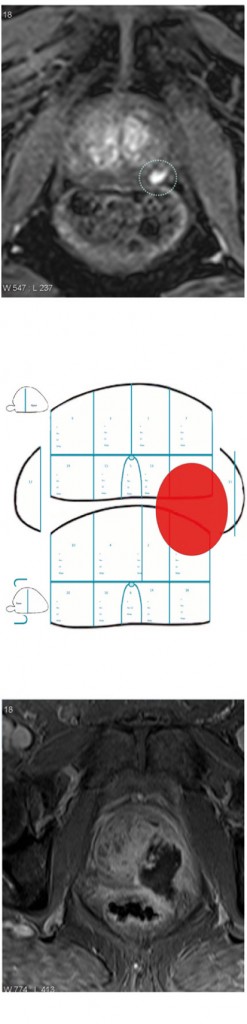
MRI for treatment planning: focal therapy
The figure left shows a left peripheral zone lesion that is clearly visible on dynamic contrast enhancement (top). This was targeted at transperineal biopsy and compared to the 24-core or 12-zone biopsy (middle) and the only cores that showed up positive were the targeted cores with 4 mm of Gleason 4+4. The patient was an ideal candidate for focal therapy based on having a very small, discrete lesion that reaches histological significance. He was treated with high-intensity focused ultrasound and the treatment effect is shown (bottom). We are seeing a revolution in the use of focal therapy, with the ability to characterise the prostate and identify areas of prostate cancer.
MRI for treatment planning: radical prostatectomy
MRI is also very useful for more traditional treatment approaches, including radical prostatectomy.
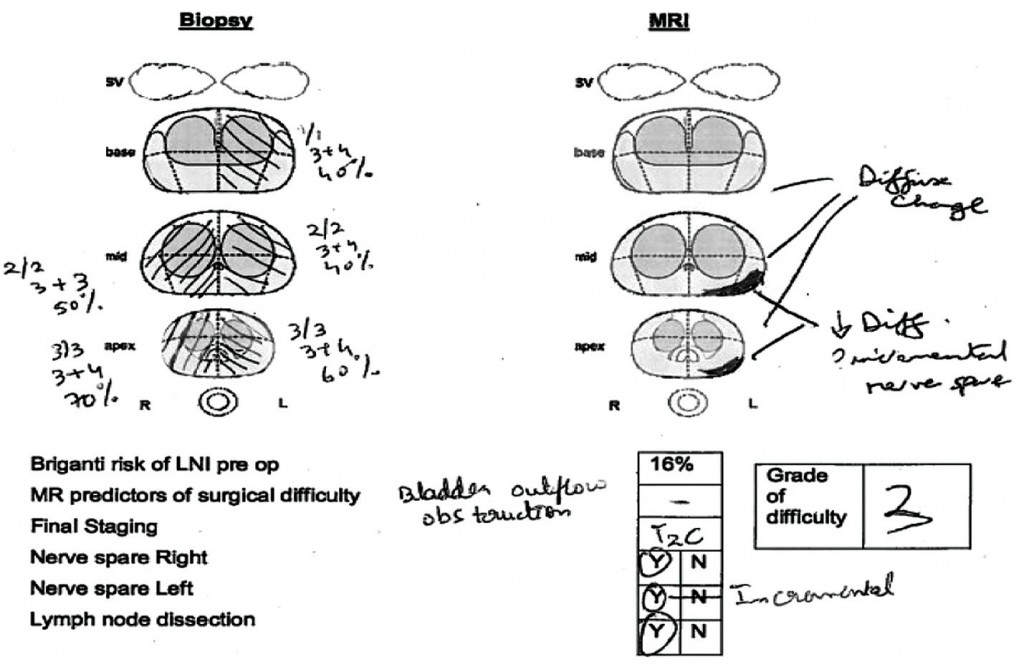
At our institution we hold robotic surgery radiology planning meetings, where robotic surgeons and radiologists discuss each case, including histological findings, patient characteristics and MRI findings. Each case is assigned a grade of difficulty for surgery (1–3), and the decision is made whether to spare the nerves or not based on MRI findings and the patient’s wishes. Plans are made for unilateral or incremental nerve spare; for the margin at the posteriolateral and anterior apex; and for how the fascia will be approached, as shown in the upper figure.
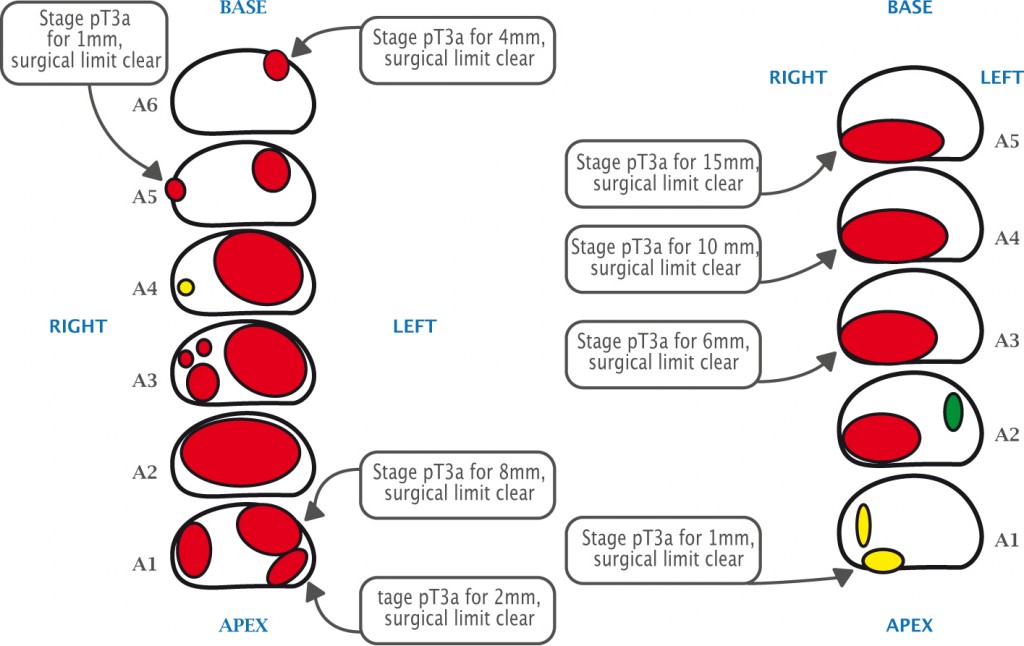
Post-operatively, the histology is correlated with the preoperative MR images. The lower figure shows two patients with quite high-volume tumours who had radical prostatectomy achieving clear surgical limits, despite the disease having spread outside the prostate.
Source: Courtesy of John Kelly, Division of Surgical & Interventional Science, University College London
Value of MRI for surgical planning
We consider that MRI adds incremental value to the use of clinical variables alone for staging prostate cancer. This value is greatest for patients with intermediate- and high-risk disease.
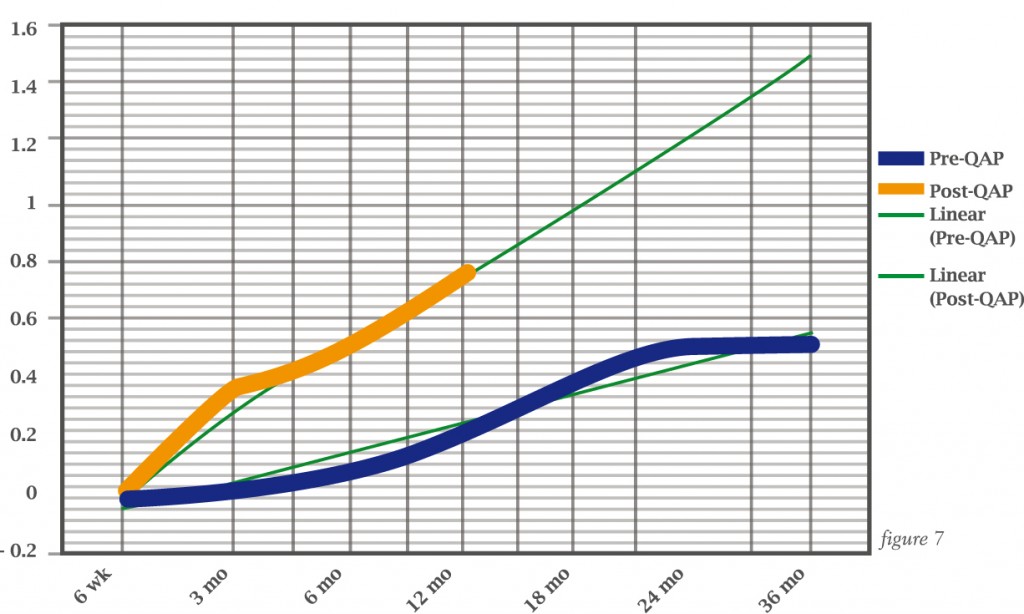
Paul Cathcart and colleagues assessed this formally during a quality assurance programme for prostate cancer surgery which included MRI, showing much better return to potency in patients in the programme compared to those treated before the programme had started (see over).
Take home messages
MRI is the best imaging modality to detect higher-risk prostate cancer. It will not detect all prostate cancer, but I consider that’s a specific advantage of MRI because, in my opinion, we don’t want to detect low-risk, low-volume, low-grade tumours.
It is important to use T2 anatomical imaging as well as diffusion and contrast enhancement.
It is important to report standard and targeted biopsies separately and discuss with radiologists. This is best done as an ongoing, learning process.We hold weekly meetings where we look at men having biopsies and focal therapy with radiologists and urologists. We feel this is important because, although we have been doing prostate MRI for a long time at University College London, there is still more to learn
Question and answer
Question
Is there a difference between CT scan and MRI for staging nodes?
Answer
The difference between CT and MRI in assessing nodal disease is less than the difference between the two techniques for assessing the prostate itself. I think it makes sense to stage, using MRI if available, because it is much better for staging the prostate. But if a patient cannot have an MRI, then CT staging of the nodes is as good. However, CT staging of the prostate is not so good.
Question
While you do need a contrast sequence at the beginning, do you consider a non-contrast MRI is suitable for follow-up?
Answer
I think it would depend on the patient. If they have a lesion that shows up well on contrast then you will want to repeat that. But if they have a lesion that shows up best on diffusion, you could potentially miss out the contrast. You can run into difficulties if you have a lot of different protocols for MRI in your system and people can get confused, so we have an initial MRI protocol and a protocol for follow-up after focal therapy, where contrast images are very important, and that can shorten the scan time.
Question
The Pinto NIH group found you have to biopsy 200 men to find one man with clinically significant disease misclassified by targeted biopsy (JAMA 2015, 313:390–397). Do you think there is still a need for standard biopsy?
Answer
In a centre with good MRI and where the radiologist is confident, a patient with a lesion scoring 4 out of 5 and where the rest of the prostate is normal, I don’t think you necessarily need a standard biopsy. But for younger men, where the prostate can look quite bright, there is diffuse enhancement and it’s hard to say, then you do need a standard biopsy.
Question
Which is the best registration system?
Answer
It’s worth learning visual registration because, whether you are a urologist or radiologist, if you’re the person doing the biopsy you should be looking at the MRI scans. Over time you will learn and get better at targeted biopsies. But there will always be some difficult lesions, and it will be interesting to see if software can help with those. The choice of software should be based on whether you offer a transperineal or transrectal service.
It’s important to use deformable registration, because with rigid registration the MR image is transferred directly across and overlaid on the ultrasound image, but the image of the prostate in an MRI scanner is different to how it looks when there is an ultrasound probe in the rectum or when it starts to swell and bleed when you have taken some biopsies.






Leave a Reply