Too toxic, too untargeted, too difficult to prove. These assumptions about chemoprevention are being challenged by important developments in the search for preventive options tailored to specific risk factors in breast cancer.
Recent years have seen some important developments in chemoprevention of breast cancer. These include the use of aromatase inhibitors (AIs) for prevention, including the MAP.3 trial with exemestane, and investigations into the best way to use targeted agents in presurgical models, such as lapatinib for HER2-positive ductal carcinoma in situ (DCIS) and metformin in insulin-resistant women who have breast cancer or are at risk of developing breast cancer.
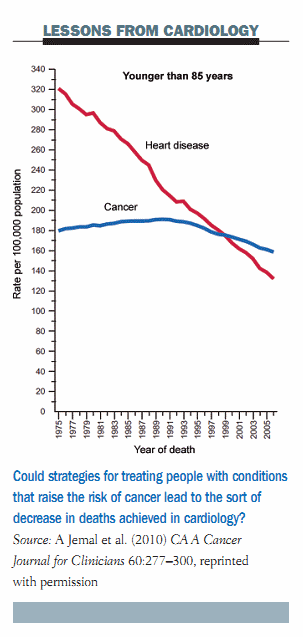
Looking at what we can learn from cardiologists, the mortality rate for cardiovascular disease in the US has fallen sharply over the last forty years compared to a relatively stable curve for cancer mortality. This is essentially due to the efforts cardiologists and other internal medicine specialists have made in the prevention of cardiovascular disease. I think we in the cancer community have to switch our efforts towards early intervention, such as treating at-risk conditions, as the cardiologists are doing with the treatment of hypercholesterolaemia and hypertension, which is translating into decreased mortality.
 Up until publication of the MAP.3 trial, two agents, tamoxifen and raloxifene, have been registered for the prevention of breast cancer in the US and Canada, associated with a 40% reduction in the incidence of breast cancer. A third compound, lasofoxifene, used for the treatment of osteoporosis, is also associated with a very significant reduction in breast cancer (see figure below).
Up until publication of the MAP.3 trial, two agents, tamoxifen and raloxifene, have been registered for the prevention of breast cancer in the US and Canada, associated with a 40% reduction in the incidence of breast cancer. A third compound, lasofoxifene, used for the treatment of osteoporosis, is also associated with a very significant reduction in breast cancer (see figure below).
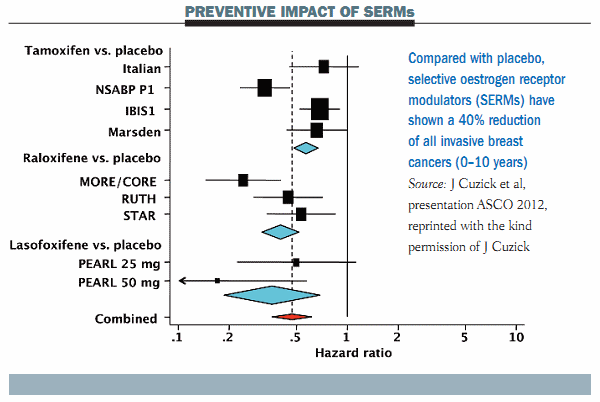 These results suggest we have several very active agents that can be given to women at increased risk for breast cancer, but unfortunately their use is associated with increased risk of important adverse events. Endometrial cancer is increased by 30–40% with tamoxifen and all selective oestrogen receptor modulators (SERMs) are associated with an increased risk of deep-vein thrombosis and pulmonary emboli. These side-effects have limited the broad use of these compounds in the clinical setting. In addition these drugs are not registered outside the US, so their use is off-label in Europe.
These results suggest we have several very active agents that can be given to women at increased risk for breast cancer, but unfortunately their use is associated with increased risk of important adverse events. Endometrial cancer is increased by 30–40% with tamoxifen and all selective oestrogen receptor modulators (SERMs) are associated with an increased risk of deep-vein thrombosis and pulmonary emboli. These side-effects have limited the broad use of these compounds in the clinical setting. In addition these drugs are not registered outside the US, so their use is off-label in Europe.
A turning point: the MAP.3 trial
A very important turning point occurred last year (2011) with the publication of the first data from the MAP.3 trial in the New England Journal of Medicine (364:2381–91). The study, which investigated exemestane in women at increased risk of breast cancer, was accompanied by a very positive editorial suggesting this was a breakthrough with this new class of agents that may represent an important step forward in the prevention of breast cancer.
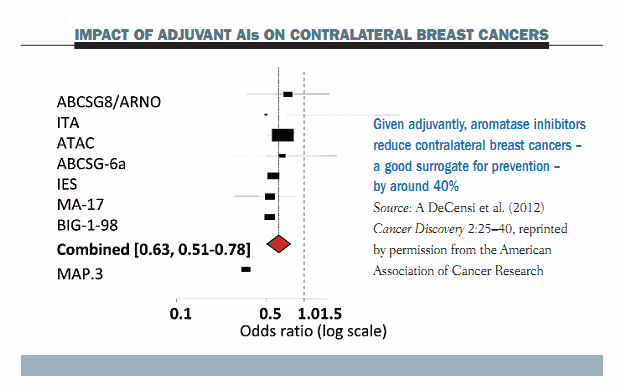
The rationale for using aromatase inhibitors (AIs) in breast cancer prevention is derived from their demonstrated effect on contralateral breast cancers. The figure below shows a Forest plot of the most important adjuvant trials with AIs, which shows clearly that the incidence of contralateral breast cancer, which is a very important surrogate endpoint for prevention, decreased by approximately 40% with all types of third-generation AIs, including anastrozole, letrozole and exemestane. Data from the MAP.3 trial show an even greater reduction in women who were treated for primary breast cancer.
 The MAP.3 trial was a double-blind trial that randomised 4560 women recruited from February 2004 to March 2010 to exemestane (25 mg/day) or placebo (1 mg/day) for five years. Study participants were post-menopausal women aged 35 years and older who had at least one of the following risk factors for breast cancer: age >60 years, Gail score >1.66%, prior intraepithelial neoplasia or intraductal carcinoma in the contralateral breast or DCIS with prior mastectomy. There were two stratification factors: the use of aspirin and the level of risk on the Gail score (<2.0 vs >2.0).
The MAP.3 trial was a double-blind trial that randomised 4560 women recruited from February 2004 to March 2010 to exemestane (25 mg/day) or placebo (1 mg/day) for five years. Study participants were post-menopausal women aged 35 years and older who had at least one of the following risk factors for breast cancer: age >60 years, Gail score >1.66%, prior intraepithelial neoplasia or intraductal carcinoma in the contralateral breast or DCIS with prior mastectomy. There were two stratification factors: the use of aspirin and the level of risk on the Gail score (<2.0 vs >2.0).
The figure below shows the main results of the trial. Starting from the first year there is a very significant reduction in the cumulative incidence of invasive breast cancer in the exemestane group compared to the placebo group. The curves tend to diverge as follow-up continues. However, the risk in the placebo arm was not as high as was probably envisioned in the study planning – at only 0.55% per year. This is slightly lower than the risk seen in earlier phase III trials – the P1 and P2 trials. The reduction in invasive breast cancer associated with exemestane was as high as 65%, with a hazard ratio of 0.35 that was highly significant (P=0.002). These data show a very remarkable risk reduction in invasive breast cancer with exemestane.
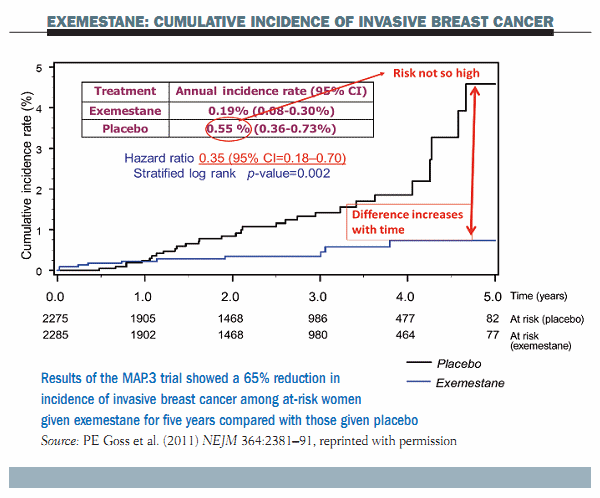 Further data showed that exemestane significantly reduced DCIS in addition to invasive breast cancer (HR 0.47). There was a favourable trend in the reduction of DCIS alone (HR 0.65), although this was not statistically significant due to the relatively small number, and also there was a borderline significant reduction of new intraepithelial neoplasia including lobular carcinoma in situ, atypical ductal hyperplasia and atypical lobular hyperplasia (HR 0.36).
Further data showed that exemestane significantly reduced DCIS in addition to invasive breast cancer (HR 0.47). There was a favourable trend in the reduction of DCIS alone (HR 0.65), although this was not statistically significant due to the relatively small number, and also there was a borderline significant reduction of new intraepithelial neoplasia including lobular carcinoma in situ, atypical ductal hyperplasia and atypical lobular hyperplasia (HR 0.36).
In terms of side-effects with exemestane, there was a significant increase of menopausal symptoms, including hot flushes, fatigue, insomnia, diarrhoea, nausea, arthritis, joint pain, muscle pain, depression and vaginal dryness. But the differences compared to placebo were generally quite limited, and only a minority of women had to interrupt treatment because of bothersome side-effects.
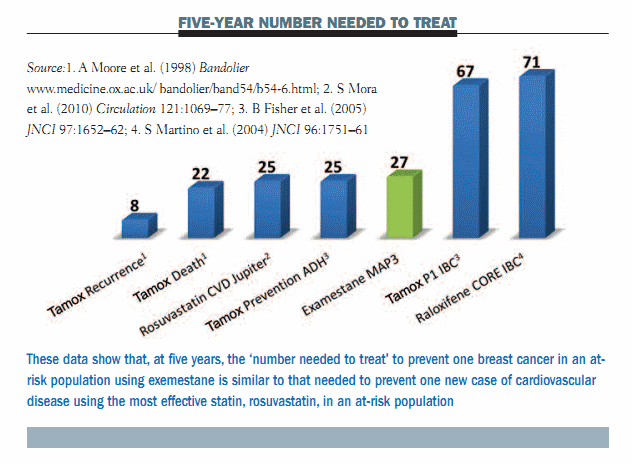
Putting these data into context, one of the main arguments against chemoprevention for breast cancer was that you have to treat a lot of women to prevent a few breast cancers. But, if we look at the data for the MAP.3 trial and use ‘number needed to treat’ at five years to illustrate the efficacy compared to other interventions, you can see that you have to treat 27 women to prevent one breast cancer. This is highly comparable to the most effective statin intervention, which is illustrated in the Jupiter trial using rosuvastatin (see figure below). The argument here is that, with an effective chemopreventive agent such as exemestane, the reduction in the risk of breast cancer in women at risk is comparable to the reduction in the risk of cardiovascular disease with statins in subjects with elevated C-reactive protein or cholesterol. The specificity or cost–benefit ratio is similar for the best preventive interventions that we have today.
 The MAP.3 trial did have some limitations. The definition of ‘high risk’ has been criticised as rather loose. The trial was also criticised for giving placebo to the comparator group instead of an active compound such as raloxifene, thereby preventing determination of the best hormonal strategy: no oestrogen at all versus the best balance between agonistic and antagonistic effect. However, a study with an active comparator would require a much larger number of patients and the cost would be prohibitive.
The MAP.3 trial did have some limitations. The definition of ‘high risk’ has been criticised as rather loose. The trial was also criticised for giving placebo to the comparator group instead of an active compound such as raloxifene, thereby preventing determination of the best hormonal strategy: no oestrogen at all versus the best balance between agonistic and antagonistic effect. However, a study with an active comparator would require a much larger number of patients and the cost would be prohibitive.
A further important criticism of the first paper (NEJM 2011; 364: 2381–91) was the lack of systematic follow-up of bone density for osteoporosis detection. The incidence of osteoporosis was self-reported and is likely to be underestimated. A very recent study published in Lancet Oncology (2012; 13:275–284) showed that exemestane was associated with significant bone loss at two years in a subgroup of women, as would be expected. This needs to be addressed in any discussion of the pros and cons of a drug intervention, as for any treatment used in the current practice of modern medicine.
Another weak point is the study’s maturity, because the data were published after only 38 events had accumulated, and follow-up was very short. Although the study is technically accurate from a statistical standpoint, it does not allow a mature judgement of the safety and long-term efficacy of the intervention. Finally, there is the problem that the study was interrupted and women on placebo were offered crossover to exemestane, so long-term follow-up including mortality data will not be possible.
A new standard of care for prevention of breast cancer
We now have a new standard for preventive care in breast cancer: in addition to tamoxifen for women at increased risk who are premenopausal, we now have exemestane (or raloxifene) for at-risk postmenopausal women. Any drug has side-effects, but this is a particular problem in cancer medicine, as any drug that interferes with cell growth is unlikely to be totally devoid of side-effects. The medical oncology community should spread the notion that most breast cancers are preventable today. Although screening has increased the detection of breast cancer at an early stage while tumours are small, there is still a risk that these tumours will eventually kill women.
Exemestane is now out of patent in the US and Europe. This means that no-one will register this drug for prevention, and therefore prescribing it will be off label, which is a barrier with current legislation. Once the efficacy of a drug has been assessed, the time has elapsed for its patent, which then makes it difficult to foster its use in clinical practice. This begs the question: How can we develop new agents to use in prevention before the patent expires?
The window of opportunity model for exploring the field cancerisation effect
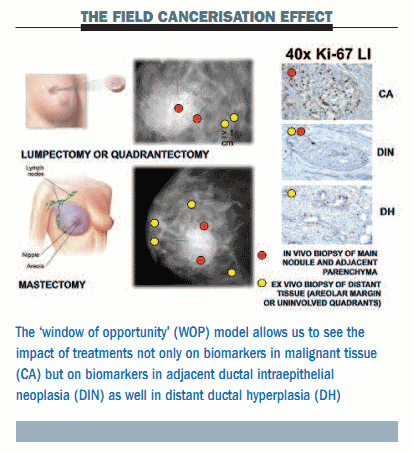
Our group has been working with the window of opportunity (WOP) model over the last few years for presurgical studies three or four weeks before surgery, to study not only the change in biomarkers in malignant tissue but also the change in biomarkers in adjacent intraepithelial neoplasia and distant ductal hyperplasia (see figure below). This model provides an elegant way to reveal the ‘field cancerisation effect’ – a term used to describe the hyperplasia and precancerous lesions that are present in tissue surrounding an actual malignant tumour.
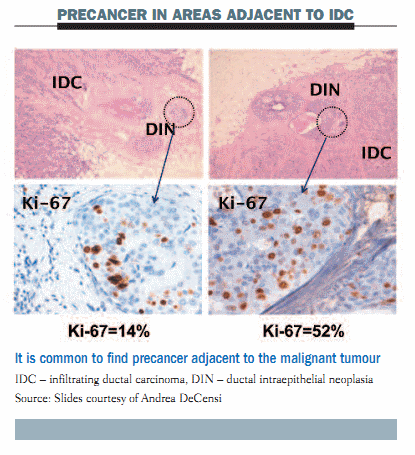
 Immunohistochemistry staining illustrated in the figure below shows that precancer adjacent to the malignant tumour – ductal intraepithelial neoplasia (DIN) – is a frequent finding. In the left upper panel, morphological assessment of the area of DCIS shows the Ki-67 (proliferation index) is 14%. In the right lower panel, the proliferation index of this area of DCIS is much higher (Ki-67 = 52%).
Immunohistochemistry staining illustrated in the figure below shows that precancer adjacent to the malignant tumour – ductal intraepithelial neoplasia (DIN) – is a frequent finding. In the left upper panel, morphological assessment of the area of DCIS shows the Ki-67 (proliferation index) is 14%. In the right lower panel, the proliferation index of this area of DCIS is much higher (Ki-67 = 52%).
 We assessed the activity of lapatinib, which is a HER1/HER2 tyrosine kinase inhibitor (TKI), in women with HER2-positive breast cancer. They were treated for three weeks with lapatinib or placebo before surgery. We first carried out a core biopsy, then treated with lapatinib or placebo for three weeks, and then performed surgery and looked at the change in biomarkers.
We assessed the activity of lapatinib, which is a HER1/HER2 tyrosine kinase inhibitor (TKI), in women with HER2-positive breast cancer. They were treated for three weeks with lapatinib or placebo before surgery. We first carried out a core biopsy, then treated with lapatinib or placebo for three weeks, and then performed surgery and looked at the change in biomarkers.
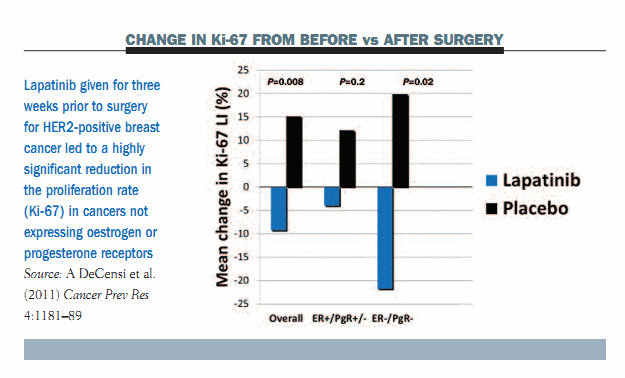
Results in malignant tissue showed an increase in Ki-67 in the placebo arm after surgery compared to the pre-surgery biopsy. In contrast, there was a reduction in Ki-67 in the lapatinib arm, which was highly significant in tumours not expressing oestrogen or progesterone receptors (see figure below).
 The most important finding was that the prevalence of adjacent DCIS was as high as 70–76% and the prevalence of distant hyperplasia was over 90%. This presurgical model revealed the high incidence of precursor conditions that are associated with tumour tissue.
The most important finding was that the prevalence of adjacent DCIS was as high as 70–76% and the prevalence of distant hyperplasia was over 90%. This presurgical model revealed the high incidence of precursor conditions that are associated with tumour tissue.
A very important question is whether we should use lapatinib for treating HER2- positive DCIS. We know that lapatinib is not as active as other anti-HER2 drugs for the treatment of breast cancer, but because it is an oral agent it may be interesting in the treatment of HER2-positive DCIS after surgery.
We wanted to understand whether lapatinib was interfering with cancer-adjacent DCIS and whether this cancer-adjacent DCIS was overexpressing HER2 or had an amplified HER2. Results showed that 90% of all HER2-positive cancer-adjacent DCIS overexpressed HER2. Lapatinib achieved a significant decline in Ki-67 in this premalignant tissue, with the reduction being highly significant in tumours not expressing oestrogen or progesterone receptors. This strengthened our hypothesis that lapatinib could be assessed as an adjuvant treatment after the resection of HER2-positive DCIS, which represents 20–25% of all instances of DCIS.
The take-home message was that short-term, pre-surgical treatment with lapatinib decreases cell proliferation in HER2-positive DCIS. We therefore believe that a phase III trial in HER2-positive DCIS is warranted.
Metformin in breast cancer prevention
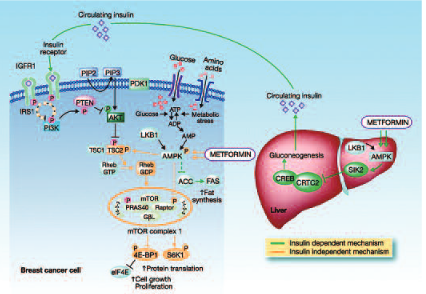
The potential use of metformin in breast cancer prevention is a very hot topic at the moment. Metformin is an anti-diabetic biguanide, which is used for the treatment of non-insulin-dependent diabetes. It is also used for the treatment of polycystic ovary syndrome in non-diabetic women. This syndrome occurs in young women and is associated with insulin resistance.
Metformin is a safe drug used by millions of people around the world. The rationale for its use in cancer prevention and treatment is that obesity is an independent risk factor for postmenopausal breast cancer. Hyperinsulinaemia – reflecting underlying insulin resistance, which is reversible with metformin – is a likely mediator of this effect. Insulin has an independent role in breast cancer development.
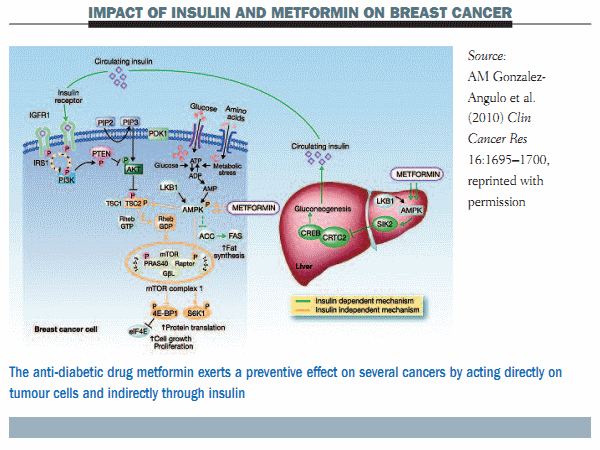
Recent data from epidemiological studies have shown that metformin can reduce the incidence of several cancers compared with other anti-diabetes agents. There are two main mechanisms attributed to metformin for this cancer preventive effect. One is an indirect effect through insulin and the second is a direct effect on tumour cells through a number of different pathways that essentially converge in the mTOR pathway (see figure below).
 A very recent study from our group showed that in a Forest plot of all studies with metformin in breast cancer, the drug was associated with a small but significant decline in breast cancer incidence compared to anti-diabetic drugs, namely insulin and sulfonylureas (relative risk 0.94). One argument is that the comparators may increase the risk of breast cancer, so it is very important to carry out clinical studies to determine whether metformin has an anti-tumour effect per se.
A very recent study from our group showed that in a Forest plot of all studies with metformin in breast cancer, the drug was associated with a small but significant decline in breast cancer incidence compared to anti-diabetic drugs, namely insulin and sulfonylureas (relative risk 0.94). One argument is that the comparators may increase the risk of breast cancer, so it is very important to carry out clinical studies to determine whether metformin has an anti-tumour effect per se.
Using our pre-surgical WOP model, we recently completed a study in 200 women with breast cancer who were treated for four weeks with metformin or placebo before surgery. We looked at the change in Ki-67 as the primary endpoint and also whether there was a different effect according to hormone receptor status and insulin resistance status.
A pivotal trial that was conducted ten years ago, the Diabetes Prevention Programme (NEJM 2002, 346:393–403), which was a primary prevention trial of metformin in subjects at risk for diabetes, showed that both lifestyle changes (namely diet) and metformin were able to decrease the incidence of diabetes. What is very relevant to our discussion here is that the effect of metformin was much greater in women who were obese, or overweight, and in those who were insulin resistant, in that they had pre-diabetes glucose levels. So, we already had important information that the effect of metformin is dependent on the metabolic milieu of the host.
The main finding of our study (JCO, published online 7 May 2012) was that there was no modulation of cancer proliferation overall (see figure below). There was a small trend to increased Ki-67 in the whole population, but this was not significant. When we stratified subjects according to insulin-resistant status, by the HOMA index, there was a different effect, with a trend to decreased proliferation in women with insulin resistance, as shown by the increase in the HOMA index >2.8. There was the opposite effect in women who were not insulin resistant, who made up the majority of the study population, with a trend to an increase in cancer proliferation. This is very different behaviour, which is represented statistically by a significant interaction that means that the HOMA index modifies the effect of metformin on cancer proliferation. A similar pattern was seen with body mass index (BMI), so women who were overweight or obese showed a decrease in cancer proliferation as opposed to an increase in women of normal weight. These findings completely resembled those of the Diabetes Prevention Programme.
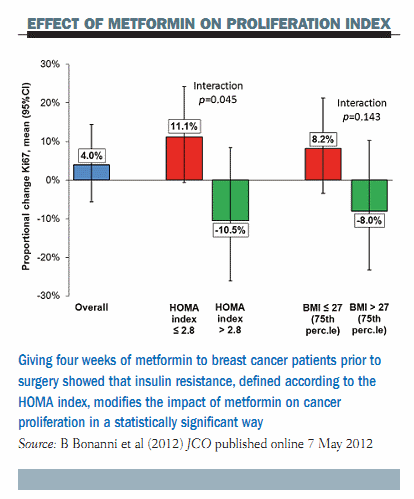 In conclusion, the take-home message here is that metformin given for four weeks prior to surgery reduced breast cancer proliferation in women with insulin resistance or with a BMI >27 kg/m2. There was an opposite trend in patients with normal insulin sensitivity or normal weight. We think that our study is very important because it proves the principle that metformin can be effective in women who have certain metabolic disorders that predispose them to an increased risk of breast cancer, namely insulin resistance or obesity. Metformin should not be recommended in older women without these metabolic disorders. A phase III trial with metformin is underway in women regardless of their BMI or insulin resistance status, but our data suggest a need for careful consideration of the study population.
In conclusion, the take-home message here is that metformin given for four weeks prior to surgery reduced breast cancer proliferation in women with insulin resistance or with a BMI >27 kg/m2. There was an opposite trend in patients with normal insulin sensitivity or normal weight. We think that our study is very important because it proves the principle that metformin can be effective in women who have certain metabolic disorders that predispose them to an increased risk of breast cancer, namely insulin resistance or obesity. Metformin should not be recommended in older women without these metabolic disorders. A phase III trial with metformin is underway in women regardless of their BMI or insulin resistance status, but our data suggest a need for careful consideration of the study population.
Bernardo Bonanni (BB), of the cancer prevention and genetics department at the European Institute of Oncology, in Milan, Italy, hosted a question and answer session with Andrea DeCensi (AD)
BB: Your take-home messages are very clear, but to get an even broader message, do you think we are approaching more targeted prevention treatment, at least in some at-risk subgroups?
AD: We live in an era of personalised medicine, and I think that prevention should follow this avenue of going towards personalised preventive therapy. We have shown a couple of examples where we can target a specific population with two interesting compounds. In the first example, we have an agent such as lapatinib, which can be used to reduce the risk of recurrence in women with HER2-positive DCIS. This is a specific population where a drug can be extremely effective. The second example is metformin, which can be used for reducing the risk of breast cancer, or for treating breast cancer after surgery in an adjuvant setting, in women with certain metabolic characteristics, such as the presence of insulin resistance or obesity. In this second instance, the population at risk is much larger, especially in Northern European or in the American countries.
BB: How do you see oncologists moving closer to cardiologists in successfully spreading prevention of breast cancer among the wider public?
AD: This is a very tough question. We should probably put less emphasis on the potential toxicity of drugs, which is intrinsic to drug treatment. I read with some disappointment the editorial in Lancet Oncology on the bone toxicity of exemestane. It gives the impression that osteoporosis is equal to breast cancer, which is absolutely not the case. We cannot compare a breast cancer prevented with a case of osteoporosis induced. The second is very easily manageable with drugs today; it is not even a disease but a risk factor. In contrast, even a small breast cancer can still be lethal. I think one approach for spreading prevention is to not give so much emphasis to the potential toxicity when you have a drug like exemestane, which is extremely effective in preventing two-thirds of breast cancers.
The European School of Oncology presents weekly e-grandrounds which offer participants the opportunity to discuss a range of cutting-edge issues, from controversial areas and the latest scientific developments to challenging clinical cases, with leading European experts in the field. One of these is selected for publication in each issue of Cancer World. In this issue, Andrea DeCensi, of the medical oncology department at Ospedali Galliera, in Genova, Italy, reviews recent achievements in the chemoprevention of breast cancer. Bernardo Bonanni, of the cancer prevention and genetics department at the European Institute of Oncology, in Milan, Italy, poses questions arising during the e-grandround live presentation. The presentation is summarised by Susan Mayor.





Leave a Reply