Understanding what drives cancer cells to break loose, travel around the body and seed new tumours and how to inhibit this process will be key to developing effective new therapies
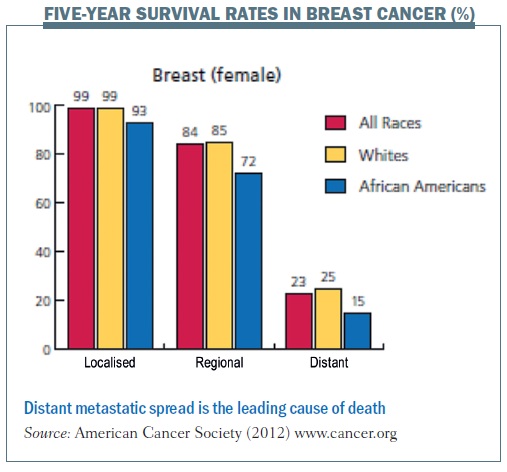
 Metastasis – the spread of cancer cells to distant organs – is one of the conditions that I primarily take care of as a clinician caring for women with breast cancer. Despite recent developments and new treatments, metastasis remains the leading cause of death from breast cancer, and five-year relative survival rates are significantly lower in patients with distant metastasis. As for other solid cancers, metastasis continues to drive mortality, motivating clinicians to figure out new ways to think about metastasis and improve survival for our patients.
Metastasis – the spread of cancer cells to distant organs – is one of the conditions that I primarily take care of as a clinician caring for women with breast cancer. Despite recent developments and new treatments, metastasis remains the leading cause of death from breast cancer, and five-year relative survival rates are significantly lower in patients with distant metastasis. As for other solid cancers, metastasis continues to drive mortality, motivating clinicians to figure out new ways to think about metastasis and improve survival for our patients.
 Traditional views of metastasis
Traditional views of metastasis
Historically, one of the ways we have thought about metastasis, certainly in breast cancer, is that a primary tumour develops and metastasis happens in a linear fashion. It is rather like stops on a train track that are going in one direction, whereby a woman develops a primary breast tumour and cells are shed from the tumour and spread in an anatomical fashion to adjacent lymph nodes. After that point, if a woman’s disease goes unchecked, she may develop disease that spreads to distant sites such as the brain, the lung or the bone. And once disease has spread to distant sites, we know that although it is potentially treatable it is certainly not curable.
This idea of the anatomical spread of the cancer, where it moves in a uni-directional fashion from the primary site to distant sites, drove our thinking about breast cancer for almost a century. It was the rationale behind radical mastectomy that for almost a hundred years was the only surgical way to manage breast cancer. The idea was to remove the primary tumour and, in order to cure the woman, to remove any of the adjacent structures that the cancer would naturally progress to – the breast in its entirety, the underlying tissue, the muscle and bone in some instances, and the axillary lymph nodes. This was the rationale behind extensive axillary node dissections, with the accompanying morbidity and upper extremity lymphoedema that many women experienced.
The conundrum is that, even with radical mastectomies, not all women are cured. Similarly, some women develop metastatic disease even with no lymph node involvement or after having an incredibly small primary tumour. And some women have extensive metastatic disease but no lymph node involvement and we can’t even find the breast primary in some patients, although biopsies of distant sites confirm they have breast cancer.
Seed soil hypothesis
This led to the thought that perhaps breast cancer spreads in a multidirectional fashion rather than unidirectionally. This ‘seed soil’ hypothesis envisages cancer as a seed that travels throughout the whole body, with the environment (or ‘soil’) as important to the spread of the seed as the properties of the seed itself. Examining breast cancer within this context is critical to understanding how breast cancer cells can spread from their primary site, where they have everything they need, go into the circulation and somehow find themselves in distant metastatic sites and thrive in some women but die in others.
Gompertzian growth
Early in his career Larry Norton showed, using very simple and elegant experiments, that cancers grow in what is called a Gompertzian fashion.
Benjamin Gompertz was an 18th century mathematician best known for his laws of mortality showing that populations grow very quickly during early stages of development and then plateau over time.
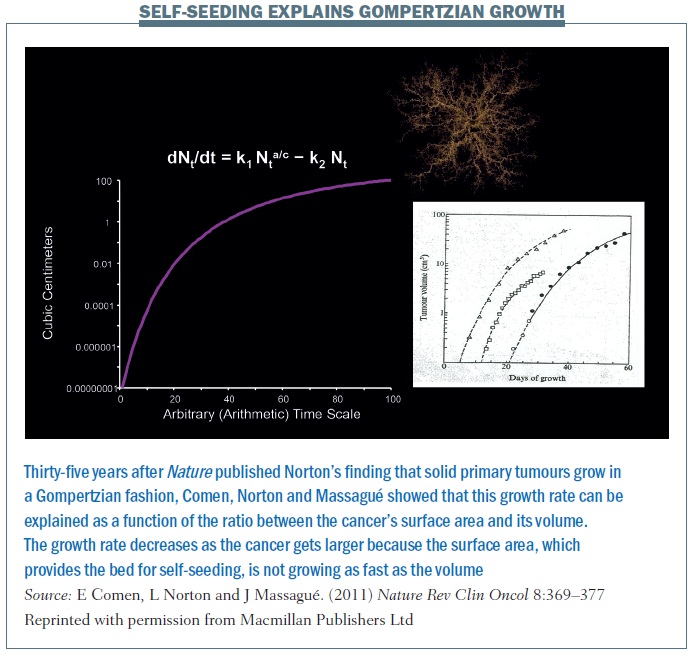
Cancers grow in a similar fashion. Each of the lines in the figure below traces the growth of different cancers. This growth pattern applies to all solid tumours – they start with a fast growth rate when they are small but, over time, larger tumours grow more slowly.
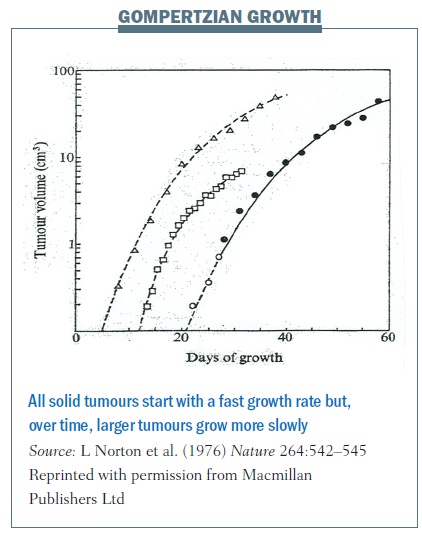 Gompertzian growth curves helped us understand tumour growth and revolutionised how we treat women with breast cancer in the adjuvant setting – that is after they’ve had surgery to remove their primary breast tumour, and when we have a window to try to cure them of their disease.
Gompertzian growth curves helped us understand tumour growth and revolutionised how we treat women with breast cancer in the adjuvant setting – that is after they’ve had surgery to remove their primary breast tumour, and when we have a window to try to cure them of their disease.
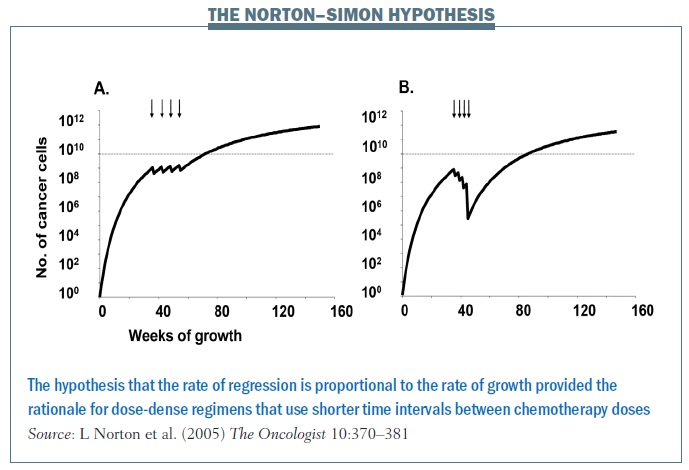
Norton and colleagues showed with the Norton–Simon hypothesis that the rate of regression of tumours is proportional to the rate of growth. So, ideally, you want to catch cancers at a shorter interval, hitting them with chemotherapy at shorter intervals so as to decrease the interval in which they can regrow. The impact of treating at shorter intervals is demonstrated in the figure above, showing chemotherapy given every three weeks in the left graph and every two weeks on the right, in what’s called a dose-dense fashion. Cancer has less chance to regrow in a shorter time interval, and continuing to give chemo in this fashion over time decreases the growth rate of the cancer and the overall tumour burden. In breast cancer this rationale led to dose-dense chemotherapy, which, in turn, significantly improved overall survival.
 Gompertzian growth curves in primary cancer growth
Gompertzian growth curves in primary cancer growth
Work by Norton and Massagué at Sloan-Kettering Memorial Cancer Center, distinguishing between primary tumour growth and metastasis, led to the hypothesis of self-seeding. Mathematical models, observations in patients and laboratory models showed that cancer cells are peripatetic, moving in a multidirectional fashion, seeding not only regional sites but also distant sites and, most importantly, the original site – the primary tumour (see below). Cancers do not grow just by cell division. If they were growing by cell division alone then they would grow in an exponential fashion, but we know from the Gompertzian growth curves that the growth of the cancers eventually plateaus, at least at the primary tumour site.
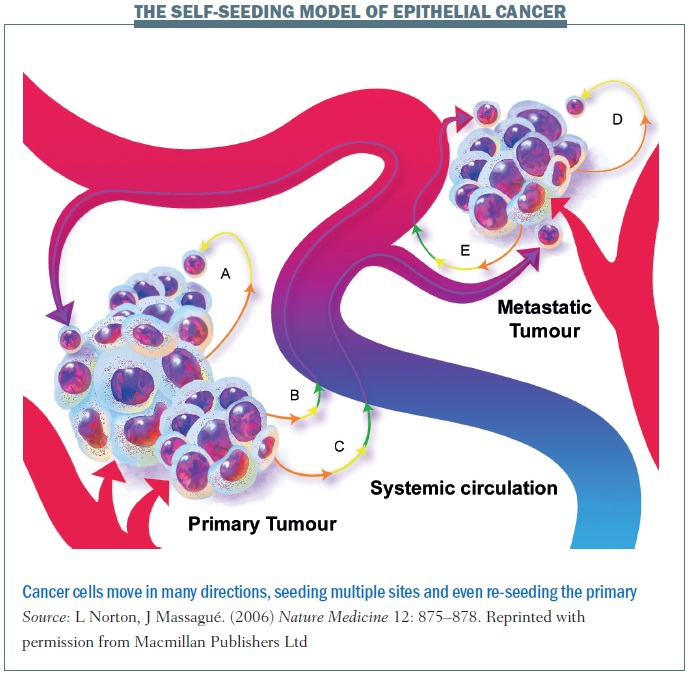 Self-seeding can take place along multidirectional paths. In pathway A, shown above, a cancer cell leaves the primary site and may travel only a short distant before returning to the primary site. The cell returns back home because that’s where its resources are and the soil where it started to grow. The cancer cell could also take pathway B, where the cell dislodges and travels into the blood system before coming back to the primary tumour. Alternatively, a cancer cell leaves the primary tumour and goes to a distant site. A cancer cell can also self-seed among distant metastatic sites. These multiple different processes remind us that the cancer spread is not simply a linear process and that the body is a dynamic system in which cancer cells can move and travel.
Self-seeding can take place along multidirectional paths. In pathway A, shown above, a cancer cell leaves the primary site and may travel only a short distant before returning to the primary site. The cell returns back home because that’s where its resources are and the soil where it started to grow. The cancer cell could also take pathway B, where the cell dislodges and travels into the blood system before coming back to the primary tumour. Alternatively, a cancer cell leaves the primary tumour and goes to a distant site. A cancer cell can also self-seed among distant metastatic sites. These multiple different processes remind us that the cancer spread is not simply a linear process and that the body is a dynamic system in which cancer cells can move and travel.
The self-seeding model essentially explains the Gompertzian growth of a primary tumour and growth at different sites (Nature Rev Clin Oncol 2011, 8:369–377; see below). The equation explains that the growth rate of a primary cancer is a function of the ratio between the cancer’s surface area and its volume. The growth rate decreases as the cancer gets larger because the surface area is not growing as fast as the volume. A larger tumour has a lower surface-to-volume ratio and a slower growth rate. This is the next step in the hypothesis, thinking about cancer not simply as one mass that is growing from the inside out but rather a mass that is growing from the inside out but also from the outside in. So the concept of the surface changes – it’s not simply one solid mass but a conglomerate of masses. If we begin to think about cancer and self-seeding as a topographical process where the concepts of inside and outside are different, this really changes the way we perceive primary cancer growth.
 Proof of self-seeding by circulating cancer cells has been demonstrated in a mouse model, injecting donor cancer cells labelled with red fluorescent dye at one site in the mammary fat pad and recipient tumour labelled with green dye at another site. Over time, the fluorescence is an amalgam of red and green, proving self-seeding by circulating cancer cells (Cell 2009, 139:1315–26).
Proof of self-seeding by circulating cancer cells has been demonstrated in a mouse model, injecting donor cancer cells labelled with red fluorescent dye at one site in the mammary fat pad and recipient tumour labelled with green dye at another site. Over time, the fluorescence is an amalgam of red and green, proving self-seeding by circulating cancer cells (Cell 2009, 139:1315–26).
Question: Coming back to Gompertzian growth, this is probably well proven by experimental models, also by observation. However, in clinical situations I sometimes see patients who have a slow growing tumour at the beginning but then growth suddenly explodes. So is the model sometimes not true?
Answer: I think the model refers to patients who have not had any treatment. There are certainly instances where cancer cells can acquire new mutations that make the disease explosive, where they’re not only self-seeding going back to the tumour but they’re exploding in metastatic sites. But at some point even explosive growth plateaus or, as often happens in these cases, the disease is no longer compatible with life.
Question: Regarding the phenomenon that cancer cells travel all the time, constantly coming and going: is this really a common phenomenon? Does it happen all the time or does it just affect a small number of patients? When you imagine cancer, do you think of it as a hive of bees, always moving about?
Answer: No. I think that there are some cancers that just stay where they are, but there are other cancers that have a tremendous ability – whether by the stem cells they have or other mutations they acquire – to move in a multidirectional fashion. What makes them able to do this is not the genes associated with mitosis and cell division but those that are associated with escaping the blood stream or migration.
Differentiating between primary tumour growth and metastasis
A series of elegant experiments took the pleural fluid from a breast cancer patient containing an amalgam of cells and introduced the cell line (MDA-MB-231) into mouse models, which were then grown into subsequent lines of mice. Results showed you could breed mice to have particular sub-clones of metastatic colonies that were either lung specific or bone specific. The figure above shows the parental cell line does not really do much when injected into another mouse. But you can sequentially breed these mice to have either 1834 cell lines or 4175 (otherwise known as LM2) cell lines, which are highly specific for lung metastasis. Similarly, shown in the red circles, you can have cell lines that have an affinity for spreading to the bone.
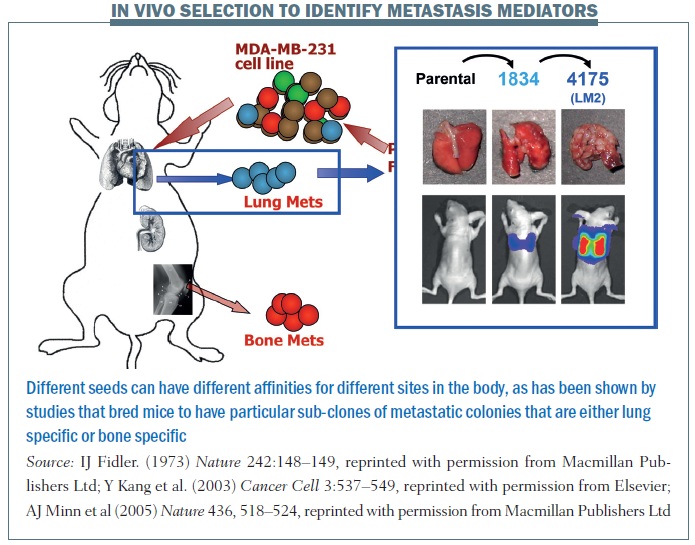 This tells us that a primary tumour is quite heterogeneous, as are metastatic colonies. What allows cells to grow in different sites is not simply a process of cell division but sub-colonies can have unique gene signatures that are associated with particular metastatic sites, so the seeding that occurs can be a function of specific characteristics of sub-colonies of cells that are in one primary tumour. Different seeds can have different affinities for different sites in the body.
This tells us that a primary tumour is quite heterogeneous, as are metastatic colonies. What allows cells to grow in different sites is not simply a process of cell division but sub-colonies can have unique gene signatures that are associated with particular metastatic sites, so the seeding that occurs can be a function of specific characteristics of sub-colonies of cells that are in one primary tumour. Different seeds can have different affinities for different sites in the body.
Question: During an operation can cancer cells go on the loose, and can each of them then self-seed wherever they land?
Answer: I wish my surgical colleagues were here to answer that question. This has been debated in the literature. I think some people worry that when you do different biopsies you may be shedding some cancer cells. To my understanding this has not been borne out in the literature; however, there are probably instances when there is some shearing of the cancer cells, and these may go into the bloodstream. One of the things that some of my colleagues are trying to do is to cryoablate cancer cells before they operate on them, not only to introduce some sort of necrotic process, but also to release some of the antigens associated with the cancer and in turn, perhaps, motivate the immune system to act as a surveillance against some of those cancer cells. There are a number of studies trying to figure out how we best deal with the primary tumour to improve survival rate.
The figure below summarises the different patterns of breast cancer growth and spread. Pathway A shows a primary cancer with some dysplasia or potentially rapid growth where the cancer is growing from the inside out but also seeding itself. Some of the cancer cells may progress to the lymph nodes (B) or out into the bloodstream and then come back to the point of origin (C), forming organ-of-origin metastases. D shows how cancer cells can spread to distant metastases from the primary. And E shows that distant metastases can go from organ to organ.
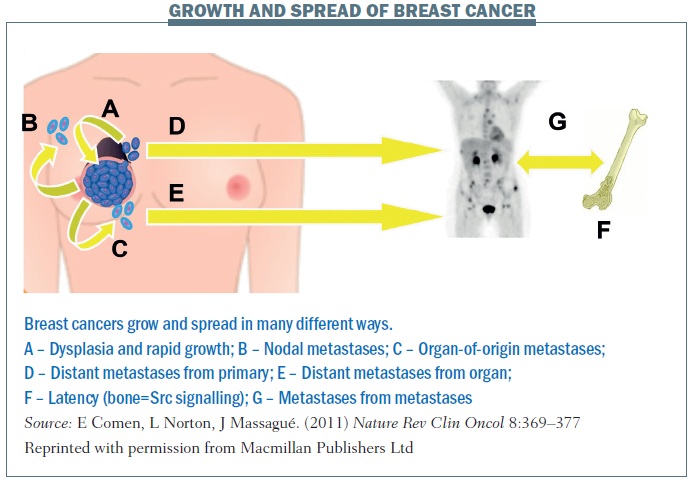 F illustrates the idea that cancer cells can spread very early at the time of diagnosis and remain latent for years. In breast cancer there are patients (often oestrogen-receptor positive) who were diagnosed 20 years ago and they think they’ve been cured, but 25 years on they come in with back pain and have developed explosive bone metastases. I’m really interested in the markers of this latency. How is it that cancer can spread at the time of diagnosis and remain dormant for so many years before reawakening? Some of my colleagues have looked at Src signalling as an important marker for bone metastatic latency and other colleagues are looking at what makes it explosive and also what protects the body from developing metastatic disease.
F illustrates the idea that cancer cells can spread very early at the time of diagnosis and remain latent for years. In breast cancer there are patients (often oestrogen-receptor positive) who were diagnosed 20 years ago and they think they’ve been cured, but 25 years on they come in with back pain and have developed explosive bone metastases. I’m really interested in the markers of this latency. How is it that cancer can spread at the time of diagnosis and remain dormant for so many years before reawakening? Some of my colleagues have looked at Src signalling as an important marker for bone metastatic latency and other colleagues are looking at what makes it explosive and also what protects the body from developing metastatic disease.
G shows spread from metastases to metastases. In patients with simultaneous neoplasms of different types, metastases from one type to the other have long been documented (J Neurosurg 1983, 774–777; Urology 1987, 30:35–38).
Finding new solutions to cancer
How does the self-seeding hypothesis help us find new solutions to cancer? Showing that a drug can shrink a primary tumour doesn’t necessarily tell you whether the drug has the capacity to reduce seeding. We need to separate treatments that are anti-mitotic, which focus only on preventing cell division, and those that are more focused on anti-seeding properties, with anti-metastatic activity – looking at what gives a tumour its mobility as well as what makes it grow. This will reframe how we think about cancer and, in turn, offer new therapeutic strategies. And it’s not just the seed, but also the soil or the microenvironment around cancers that is incredibly important. What is it about that microenvironment that allows the cancer cells to grow?
Question: What parameter do you have to measure for anti-seeding therapies? Is there any clue about what you measure to see if a therapy is really anti-seeding?
Answer: The problem is trying to develop models we can use in the lab. The obvious answer would be to see if an agent works in patients, but we don’t do that right away. We always start with models in the laboratory in which we can study seeding properties and not just shrinking the primary tumour. There are a number of different molecules that we believe are associated with seeding, and we are trying to study these in the laboratory to see if we can develop models that are able to show seeding tendencies. Massagué’s laboratory has developed mouse models with cell lines that are lung metastatic or bone metastatic that we can use to develop drugs to decrease bone metastasis or lung metastasis.
Interplay between oncology and immunology
There is an interplay where the seed interacts with the soil, and I am interested in how the immune system plays a role in either promoting or inhibiting metastasis. In particular, I am looking at how neutrophils can help promote primary tumour growth in some cases, while in others they can decrease metastatic seeding.
An example in a mouse model, also shown in patients, of how the microenvironment can play a key role in seeding is the finding that a subset of neutrophils decreases lung metastasis. Control mice injected with breast cancer cells through the tail vein developed lung metastases. We took out circulating neutrophils from a similar population of mice, with lung metastases and primary tumours, and found these circulating neutrophils have cytotoxic properties to cancer cells. We gave these neutrophils to mice injected with cancer cells that we would expect to develop lung metastasis and found a reduction in the burden of lung metastases (Cancer Cell 2011, 20:300–314).
Was this just a function of the neutrophils, so the neutrophils don’t need to be in contact with a cancer in order to reduce seeding? We did the same experiment with G-CSF-stimulated neutrophils that had had not been primed by having previous contact with cancer cells, and found they had no effect in reducing metastatic burden.
But what about patients? We took blood from healthy women who were accompanying breast cancer patients to clinic visits and compared it with blood from women with ductal carcinoma in situ – preinvasive lesions. We also took blood from women newly diagnosed with breast cancer with an intact primary tumour and no evidence of metastatic disease. We spun out their neutrophils and found the neutrophils from breast cancer patients were able to kill twice as many breast cancer cells in a petri dish compared with neutrophils from healthy volunteers.
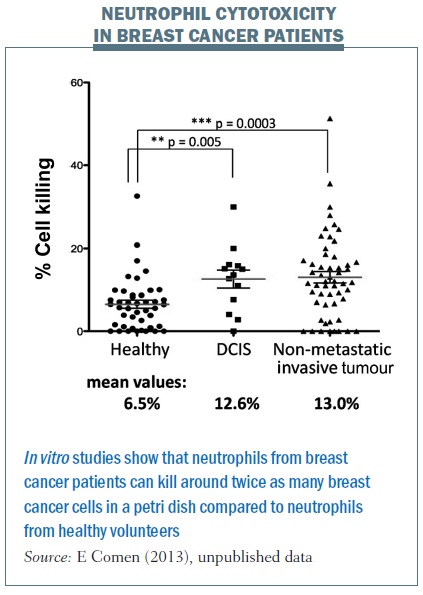 This tells us that some breast cancer patients have neutrophils able to kill cancer cells, which triggers an incredibly exciting thought process. It tells us that it is not just about the cancer itself, but the immune system is crucial in helping to fight breast cancer and potentially breast cancer seeding. I am interested in the neutrophils themselves and also what may be in the serum to help promote neutrophils to either kill cancer cells or alternatively to promote tumour growth. We are studying what these serum factors are and which factors activate neutrophils to kill breast cancer cells as opposed to helping them grow.
This tells us that some breast cancer patients have neutrophils able to kill cancer cells, which triggers an incredibly exciting thought process. It tells us that it is not just about the cancer itself, but the immune system is crucial in helping to fight breast cancer and potentially breast cancer seeding. I am interested in the neutrophils themselves and also what may be in the serum to help promote neutrophils to either kill cancer cells or alternatively to promote tumour growth. We are studying what these serum factors are and which factors activate neutrophils to kill breast cancer cells as opposed to helping them grow.
Summing up
Redefining the problem of cancer spread requires understanding the flow of metastasis as not simply a result of linear anatomy, but also as a dynamic, multidirectional process. Primary growth may be not only a process of cell division but potentially also self-seeding. Laboratory and clinical evidence suggests that metastasis happens not only in a linear fashion but also by distant and self-seeding of both the primary and metastatic sites. Metastasis may be a function of specific signatures within the heterogeneous cell population of a primary tumour. Prognosis is a consequence of the inherent biology of a cancer, which may not always be reflected in the number of lymph nodes involved or the size of the tumour.
We need to continue to understand the biology of the cancer cells that we’re studying, not just as a conglomerate mass, but whether they have aggressive or non-aggressive properties.
To develop new therapies we need to remodel the way we think about treating breast cancer and focus not just on cell division but also understanding the seeding processes and the microenvironment.





Leave a Reply