
Will pathology services need a serious overhaul if they are to deliver the accuracy required for precision medicine? Two European initiatives are trying to build the case for change in this least visible of cancer disciplines.
The shift towards personalised medicine has placed a new premium on detailed and accurate pathology reports to inform treatment decisions. But health systems and individual institutions have often been slow to understand the implications and invest in the necessary training, quality assurance and organisational changes to ensure their pathology services are up to the job. This issue was first highlighted because of concerns about the quality of data being used for clinical trials. However, attention is now beginning to focus on the implications of poor quality pathology for everyday clinical decision making.
Recent months have seen two important initiatives to raise standards of cancer pathology across Europe. The first, launched at a meeting at the European Commission this February, is focused on the particular problems associated with rare cancers. The second addresses the pathology challenges of one of the most common cancers, with the launch of the Optimal Pathology Manifesto at the European Breast Cancer Conference in March.
A benchmark for breast pathology
Emiel Rutgers, head of surgery at the Netherlands Cancer Institute, led the launch of the breast pathology manifesto on behalf of the European Breast Cancer Council (see ecco-org.eu/Events/Past-conferences/EBCC9/Manifesto.aspx). He told a large audience that it is not the intention to ‘bash’ pathologists, but there is a large variation in the quality of pathology around Europe (and the world) that must be addressed if the full range of questions from colleagues about who to treat, and how, can be answered.
Radiologists need correlations between what they and the pathologists see; surgeons need to know whether surgery is needed and the extent of an operation; medical oncologists want information on risks of relapse and suitability for drug treatments; radiotherapists also need local relapse risk data; and geneticists ask for hereditary risks. With breast cancer having such a large heterogeneity in types – and taking patient preferences into account – the implications of misjudging say whether a tumour is associated with a HER2 mutation, or whether breast conserving surgery is suitable, can be profound.
The manifesto, which is currently out for consultation, is not a guideline, Rutgers stressed. It sets out in two parts what a breast cancer pathology service should provide, but does not detail the processes as a guideline would. The first part is a list of parameters, which are the usual histological reports on type, grade, size and operative margins, and tests for hormone receptor and HER2 status. Also included are vascular invasion, multifocality/centricity (whether there are multiple tumours in one or several breast quadrants), and Ki67, which is a marker of cell proliferation. In each case, the reason why these parameters are important is noted – one of the aims of the manifesto is to give people information they can use to become more informed about their own or a family member’s pathology report.
“One aim of the manifesto is to help people become more informed about their own pathology report”
The second part itemises the organisational factors that can deliver an optimal service, divided into what is ideally needed at individual, departmental, hospital and national health system levels. By presenting both technical and organisational factors, the manifesto team hopes that clinicians, policymakers and patient groups will have a benchmark that can help them make judgements about the overall quality and standing of breast cancer pathology, not least from the point of view of the patient and the priority given to the specialty in health services.
The manifesto also aims to focus attention on variability in the quality of pathology reports, as this can impact heavily on the choice of treatments. Poor quality pathology can point to the need for improved organisation – more specialist expertise, or better multidisciplinary working and workforce development. While so-called ‘inter-observer’ variability has been reported in cancer pathology for many years, there are few large studies about its prevalence and consequences – and gathering more data is one of the manifesto’s recommendations.
In his presentation at EBCC, Rutgers mentioned one such inter-observer comparison study, conducted by colleagues at the Netherlands Cancer Institute, which looked at differences in the pathology reports of local node-negative breast tumours that were first assessed locally and then by central review (Ann Oncol 2010, 21:40–47). The findings show substantial differences. For example, central review changed the tumour grade in 28% of patients (with a 35% variation in assessment for grade 2 tumours), and 21% of tumours were wrongly classified as HER2-positive. While these results are broadly in line with findings of other reports, this study went beyond documenting inaccuracies to look at the impact this had on selecting patients for adjuvant therapy. When various guidelines and tools (e.g. the Dutch guidelines, St Gallen guidelines, and Adjuvant Online!) were applied after central review, the results showed that as many as one in seven patients had been put into the wrong risk classification.
“The results showed that as many as one in seven patients had been put into the wrong risk classification”
Some variation is inevitable, as Cancer World has reported (Nov–Dec 2012). HER2 testing, for example, is currently not an exact science, and pathologists will vary in assessments of grade and size. But as Tibor Tot, head of laboratory medicine at Falun Central Hospital in Sweden, and a manifesto team member, comments, very few countries are tracking the variability. “We do now report ER/HER2 test results to a central agency, but even in Sweden the variation is substantial between labs – it can be about 20–30% for, say, ER results, although this variation could be normal. But if you don’t monitor it, the variation could be larger and of possible concern.” Tot, who is from Serbia and has worked in eastern Europe, says there can be major differences in services that are not focused on breast pathology, noting that general pathology departments reporting few cases cannot possibly perform at the level of a major teaching hospital.
He points out that there is much less comparative data on the basic pathology reporting of type, grade and tumour size than on immunohistochemical and molecular tests for ER and HER2 status, as many laboratories take part in external quality assurance schemes for these tests. “In Sweden we monitor the tumour grade variations but not the tumour size – so we don’t know for sure whether all the studies we have done on breast cancer were made on the right sized tumours,” he says.
Latest data recently reported from a major project shed more light on how variations in basic parameters can affect patient treatments. The Sloane Project – a UK audit of non-invasive breast cancers and atypical hyperplasias detected in the breast screening programme – collected data from 8,313 patients with DCIS (ductal carcinoma in situ) from 2003 onwards. It has found that many women had a mastectomy for DCIS either as a result of failed breast conservation surgery (799 women, mostly where disease extent had been underestimated) or for tumours that turned out to be smaller than 20 mm in diameter and so should normally have had a lumpectomy (510 women). In total, nearly half of mastectomies were in these two groups (EJC online 26 May 2014).
Jeremy Thomas, a consultant breast cancer pathologist at Western General Hospital, Edinburgh, who led the study, says that analysis of the data shows wide variations in mastectomy rates between hospitals. He believes variations in the quality of the pathology are partly to blame. “There are two areas I feel are probably critical in these DCIS cases. One is the quality of the multidisciplinary assessment, in particular between imaging and pathology, where the pathologist is adding crucial information on whether the lesion is indeed DCIS or not, the grade of the lesion and the extent of suspicious calcification. The second is speculation – there are probably differences in zeal among units for carrying out breast-conserving surgery.”
Involvement in the team
All cancer pathology depends heavily on rigorous multidisciplinary communication and constant evaluation of how teams are assessing critical parameters such as tumour size and grade, says Thomas. “Above all, it just shouldn’t be an option whether to go to the multidisciplinary meeting – we should always aim to do so,” he says. And as Rutgers told the EBCC manifesto session: “It’s about precision medicine – no guideline can beat a multidisciplinary board meeting with optimal pathology to hand.”
“It just shouldn’t be an option whether to go to the multidisciplinary meeting – we should always aim to do so”
Thomas argues that, even where there are no large teams with specialist consultants, it is possible to organise part-time specialists in tumour types. He adds, however, that small services probably won’t have the capacity that larger centres have to hold weekly breast pathology team meetings, where the intricacies of say a rare phyllodes tumour may be discussed, often with trainees in attendance, which in this tumour could have important implications for recommendations on the extent of surgery.
While many pathologists and their services are subject to external assessment and accreditation around Europe, a review this year in the UK is highly critical of the lack of accountability the country’s pathology services have to the health system and to patients. The Pathology Quality Assurance Review was prompted by problems with breast cancer tests at an English hospital over several years where a number of women should have received different treatment, and about 80 women were recalled, while some may have died because of the mistakes.
While noting that the UK and the Netherlands were the first European countries to introduce a laboratory accreditation scheme for pathology, the review says the current system “relies almost entirely on professionalism and goodwill.” It calls for pathology to be visible to patients and accountable to commissioners, especially given the rapid advances in the field and the variation among services, and for more assurance of the clinical effectiveness of pathology. Many recommendations are made that could also be applicable across Europe, such as for standardisation to cut variations in practice, improving training, sharing of error reporting, and updating accreditation to show clearly which laboratories are doing more than what has been minimally acceptable.
The European breast cancer pathology manifesto also includes generally applicable organisational actions, but notes that such calls need to be realistic given that pathology is currently suffering from a shortage of specialists and huge workloads around Europe.
Attracting more doctors to take up pathology would certainly seem to underpin its future and there is no shortage of exciting issues, in particular the major advances in molecular pathology for which more specialists are urgently needed to both treat cancer and research its treatments. Technology such as digital imaging of specimens and telemedicine can help greatly with the problems of lack of specialised expertise in remote clinics.
Rare cancer pathology
If shortage of specialist pathologists, especially in remote clinics, is a problem in the field of breast cancer, the challenge is considerably greater when it comes to cancers that are much less common.
The particular issues that pathologists face when encountering rare cancers such as sarcomas have been explored in a previous issue of Cancer World (May–June 2013). Data from studies show that a large number of diagnoses of sarcoma in Europe are wrong, and that without robust second opinion systems, opportunities to provide the correct treatment can be missed and in some cases irreversible mistakes made.
Not only is sarcoma rare, but it also comprises many different subtypes that only those with expertise and a sufficient volume of cases should diagnose.
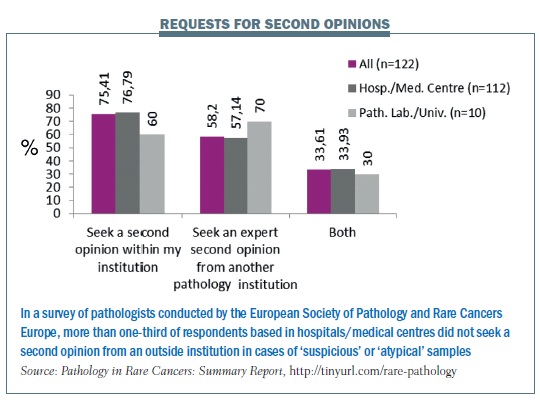
A survey carried out by Rare Cancers Europe in 2012 (Pathology in Rare Cancers International Survey, http://tinyurl.com/rare-pathology) found low standards in pathology in eastern and southern Europe in particular, and a need for more education and training. Only two countries (France and Sweden) currently have mandatory referrals to expert centres.
This February, in an effort to draw attention to these worrying findings, and build political support for action, a consensus on rare cancer pathology was launched at a meeting at the European Commission, hosted by MEP Zofija Mazej Kukovič. This was a joint initiative between Rare Cancers Europe, ESMO and the European Society of Pathology, and was preceded by an all-day meeting at which each group of rare cancers – sarcomas, rare urological and lung cancers, neuroendocrine tumours and so on – was discussed from a pathology perspective and parameters agreed.
 The co-chair, Paolo Casali, a medical oncologist and sarcoma specialist at the Istituto Nazionale dei Tumori in Milan, talked at the launch meeting of the ‘tragedy’ that 30–40% of diagnoses of rare cancers could be wrong, if sarcoma studies are anything to go by. He pointed to differences in survival of patients with sarcoma across Europe. “The pathological diagnosis may be one of the crucial factors underlying these discrepancies,” he said.
The co-chair, Paolo Casali, a medical oncologist and sarcoma specialist at the Istituto Nazionale dei Tumori in Milan, talked at the launch meeting of the ‘tragedy’ that 30–40% of diagnoses of rare cancers could be wrong, if sarcoma studies are anything to go by. He pointed to differences in survival of patients with sarcoma across Europe. “The pathological diagnosis may be one of the crucial factors underlying these discrepancies,” he said.
“30–40% of diagnoses of rare cancers could be wrong, if sarcoma studies are anything to go by”
Angelo Dei Tos, the group’s other co-chair and head of pathology and oncology at the General Hospital in Treviso, Italy, gave a vivid example of a patient who died after being misdiagnosed with GIST, and given escalating doses of Glivec, when in fact she had another type of sarcoma that should have been treated differently.
The overall consensus for rare cancer pathology, said Casali, is on three points: referral to expert pathologists is crucial; there should be networks that arrange referrals; and pathologists should be in multidisciplinary teams where they are challenged to do their best work. Achieving all three, he accepted, is not always easy.
Pathologists with expertise in rare cancers are generally in short supply, and it can be hard to sustain expert multidisciplinary teams without high levels of centralisation. And as Anastassia Negrouk, from the EORTC (European Organisation for Research and Treatment of Cancer), pointed out, there are few reference centres that have the quality controls, open access to data and networking in place that the EORTC would like to see.
Furthermore most patients and doctors have little knowledge of those that do exist, which can lead to delays in referral. While the potential for setting up European reference networks has been enhanced by the cross-border healthcare directive, which came into force in 2013, it was noted that questions remain over how they will be funded.
In the meeting of experts that preceded the Commission event, many complex issues about each family of rare cancers were discussed, which will form the basis for a position paper that presents expert consensus across rare cancer pathology. Getting action around this consensus will, however, require political pressure if rare cancer care is to be better resourced and readily available in more places – not least, a change in attitudes that too often see pathology as a cost centre rather than an essential factor in quality care.






Leave a Reply