
The concept is attractive, the results in some patients have been stunning, but what will it take to extend these impressive responses to wider groups of patients? Peter McIntyre reports.
There is a growing sense of confidence that immunotherapy will deliver long-term survival to an increasing number of cancer patients, including some people with advanced and metastatic disease.
The percentage of cancer patients with advanced melanoma who have a prospect of long-term survival roughly doubled from around one in five on ipilimumab to more like two in five on the newer range of checkpoint inhibitors that combat the ability of tumours to switch off the T-cell response. Early data from patients with other tumours, such as non-small-cell lung cancer (NSCLC) and bladder cancer, also indicate important survival benefit among patients who respond.
Trials and clinical use suggest that a percentage of patients can be kept alive in the long term. Some patients with advanced melanoma are still alive ten years after they started treatment with ipilimumab, and the newer anti-PD1 and anti-PD-L1 therapies are expected to extend this still further.
For patients who do respond, the impact on quality of life can also be dramatic. One study showed quality of life for patients with advanced NSCLC even rising above the levels for the general population.
It is predicted that combinations of more than one immunotherapy, and combinations of immunotherapies with chemotherapy, targeted therapy or radiotherapy will improve survival in an ever wider number of cancers, though potentially at the cost of serious toxicity.
Immunotherapies work across a wide range of cancers, but only for a minority of patients
The sense of excitement is palpable. There are currently more than 1,200 trials for cancer immunotherapies listed on clinicaltrials.gov, mainly for therapies that seek to block the tumours’ defences, but also for chimeric antigen receptor (CAR) therapies that engineer T cells to attack the tumour and are designed provide a memory effect and long-term protection.
However, there are significant obstacles to developing immuno-therapies that work for more patients in a wider range of cancers.
The European Medicines Agency and the Brussels-based Cancer Drug Development Forum convened an international summit in February 2016, where leading global experts in immunotherapy discussed how to amass the evidence that will convince regulators and health technology assessment (HTA) bodies to approve these treatments for more indications and patients. The meeting attracted researchers, clinicians and patient representatives, from Europe and North America, with strong representation from pharmaceutical companies and the participation of the US regulators, the FDA.
Much of the current excitement is focused on a class of drugs that inhibit PD-L1 (programmed death ligand 1), which plays a key role in protecting cancers from attack by the body’s immune system. The protein, which is expressed by cancer cells, switches off T cells by binding to the PD-1 marker on the cell’s surface.
In a keynote address, Ira Mellman, Vice President of Research Oncology at Genentech, said that this new class of drugs represents a paradigm shift in treatment. “What you can achieve, albeit as yet for a relatively small number of patients, using immunological approaches is unlike almost anything I have seen in the past with respect to other types of cancer therapies in major disease indications.”
But he also emphasised how much there is still to learn about how these therapies function and in whom. The current excitement, he argued, is being driven largely by clinical data, which has outpaced basic scientific understanding of how these therapies work.
“Exciting as these agents are, they really only benefit a small minority of patients in all but the most responsive indications such as melanoma, which can be 30–40%. Almost all the other indications are in the order of 10–20% as single agents. Well tolerated and highly effective, but not everybody benefits.”
Fast and furious
Paolo Ascierto from the Istituto Nazionale Tumori in Milan argued for adopting a “fast and furious” approach to testing combinations and identifying the right sequencing, dosage, and length of treatment for the right patients to increase the response rate and reduce the impact of side-effects. The potential prize, at least in advanced melanoma, he says, could be a further doubling of the proportion who survive for the long term, from 40% to 80%.
Traditional approaches to trial design, it was widely agreed, are simply too slow and inflexible. As Samir Khleif, Director of the GRU Cancer Center, in Augusta, Georgia, put it: “The elephant in the room is the 300 years and multi trillion dollars [it could take] to be able to reach where we want to reach.”
For patients with advanced cancers, the “fast and furious” approach certainly resonates with their need for urgent progress. The challenge is how such an accelerated approach can deliver the level of evidence on efficacy and value required by regulators and payers.
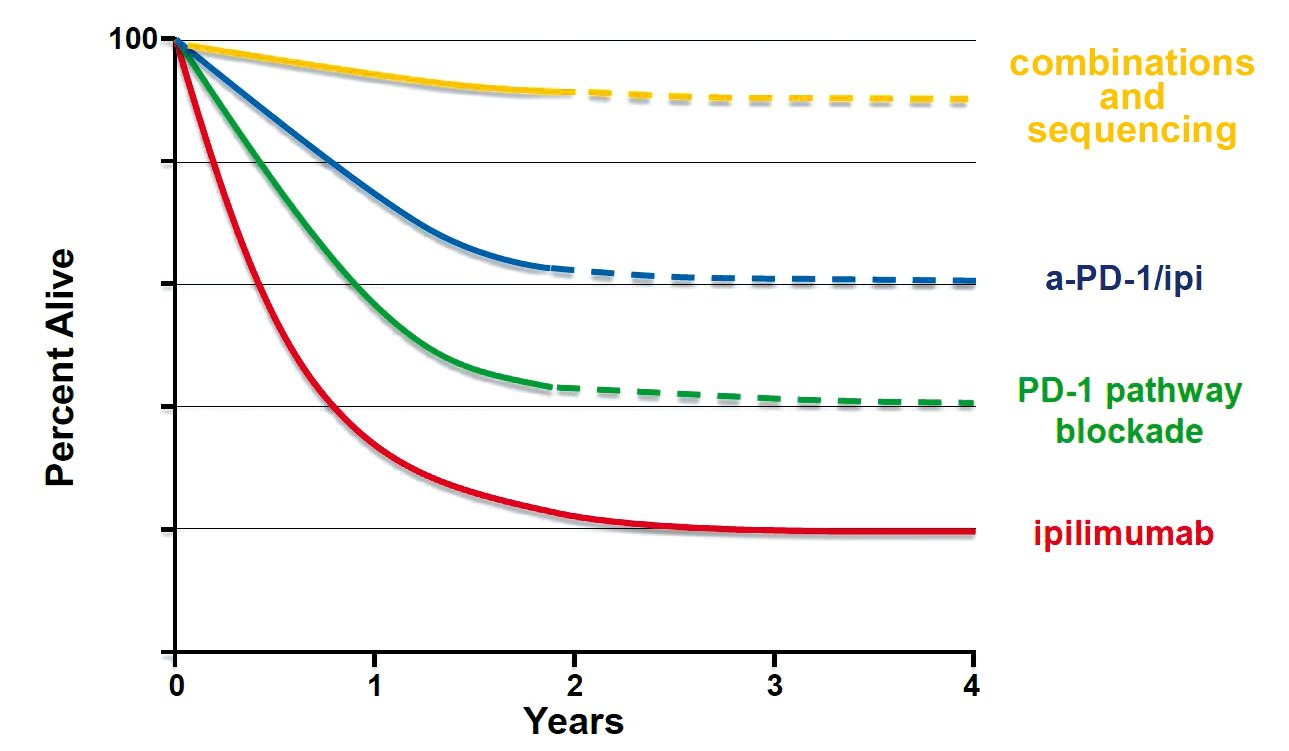
Adapted from Walter J Urba, ASCO 2013
Balancing ethics and evidence
Immunotherapy agents are already being trialled in combination with one another, and with chemotherapy, radiation therapy and small molecules, with the potential for much higher response rates, especially in those who have low levels of PD-L1.
Many early results continue to both excite and impress, despite some serious toxicity issues. An ongoing combination study in patients with non-small-cell lung cancer, for instance shows that almost 40% of patients who are PD-L1 negative respond, compared with just 5% on monotherapy.
But these early indications of success raise problems of their own, as Ramy Ibrahim, immunotherapy clinical vice-president for AstraZeneca, pointed out. “It will be very difficult to enrol to a monotherapy arm when the early data is suggestive of limited activity of monotherapy. When we start thinking of a randomised trial with a monotherapy arm of a CTLA4 inhibitor, patients are reluctant to consent to such a study design,” he said.
Vagn Erik Jesperson, a 13-year survivor of NSCLC and co-founder of the Danish Lung Cancer Patients Association, posed a question to the meeting about the ethics of conducting trials where early results had shown such major differences between the trial arms. “How do you feel about that when you make [such] clinical trials?” he asked.
Using adaptive designs that allow a trial arm that was proving to be inferior to be dropped was seen as one way to mitigate this problem. There would be a cost, however, in terms of losing data that could help get approval and reimbursement for the novel therapy.
Khleif suggested that learning from pre-clinical research in the lab will help rule out combinations that are unlikely to work. “The biology is very important. We need to think about the optimum response before we put millions of dollars into what we need to take it forward and invest.”
Ibrahim agreed that animal models can guide the search for combinations and dosages. “The biology is very complex when we start looking at combinations. We need to find the relevant animal model that can help us, so we don’t end up spending 300 years trying to figure out which is the right combination to take into the clinic.”
But as Bernard Fox, from the Earle A. Chiles Research Institute in Oregon, pointed out, mouse models can only take you so far. “The biology of the tumour in a mouse and its interaction with the immune system is fundamentally different from what you find in a human. I would encourage regulatory agencies and companies to be thinking about innovative clinical trials with small numbers of patients studied aggressively and in depth to learn as much as you can about what happens.”
More investment is also needed in basic science to better understand the mechanisms of the immune response, said Fox, singling out in particular the need for a microbiome equivalent of The Cancer Genome Atlas (TCGA), which could help define the role played by the body’s microbiota.
Finding the right endpoints
Choosing the best indicators, or ‘endpoints’, to demonstrate, within an acceptable timescale, that a novel therapy is more effective and brings more patient benefit than its comparator is always tricky. With immunotherapies, those problems seem to have become even tougher, because of the unique way in which they kick in.
Overall survival – showing that patients live longer – has traditionally been the gold standard in pivotal clinical trials. However, as new therapies are trialled in different doses and combinations, and trials often allow patients on one arm to cross to another if they progress, once survival times go beyond 18 months it becomes extremely difficult to demonstrate that differences in overall survival are attributable to a particular treatment.
To get around this problem, trials of other types of cancer therapy are increasingly using progression-free survival as a surrogate for overall survival. This doesn’t work so well with immunotherapies, as checkpoint inhibitors often elicit an initially slow (or no) reaction followed by a long survival curve for a percentage of patients.
The RECIST criteria for judging progression by monitoring the size of tumour lesions is of little use in these circumstances. Even experienced clinicians can be fooled, for instance, when a head and neck cancer treated with pembrolizumab appears to grow alarmingly before an equally dramatic regression.
Current trials of immunotherapies are therefore taking place without any agreement on what should be used as a surrogate for overall survival, at least in advanced disease.
The story of ipilimumab is instructive. The anti CTLA4 antibody passed through three different companies and underwent six clinical trials over 10 years before it was shown that it extended patients’ lives. The problem was finding a clear parameter of response that could be documented. The pivotal study was expected to last three years but Bristol-Myers Squibb (BMS) had to extend it a further 1.5 years to gather enough data for marketing approval.
Tai-Tsang Chen, Executive Director of Global Biometric Sciences (part of BMS), says it might be sufficient in future to follow only the earliest trial entrants to get long-term follow up, and use ‘milestone survival’ for the rest to indicate survival rates ‘x’ years after treatment begins.
Finding effective endpoints for immunotherapies “presents one of the most important challenges we have as a field,” says Dan Cheng, who leads work on immunotherapy at Genentech. “These drugs have a powerful effect on overall survival, but probably all of these studies will be confounded by massive use of immunotherapies in multiple lines.”
He talked about the “immune-modified RECIST criteria” Genentech has introduced into randomised trials to study detailed efficacy patterns and get a sense of whether they predict survival. “One of the big questions would be to work in partnership with health authorities to the point where we all feel confident enough with a new endpoint like this to use it for actual approval in the future,” he said.
Quality of life
David Cella, Director of the Institute for Public Health and Medicine at Northwestern University, Chicago, highlighted the importance of measuring quality of life endpoints. As survival gets longer and harder to measure, the focus will turn more to the quality of that survival.
Long-term successful treatment carries its own quality of life challenges, he added: “It is one thing to have short-term horrible diarrhoea – it is quite another to have it for two or three years. Some of the long-term treatment can be intolerable.”
He also pointed to a potential correlation between quality of life and survival. “In advanced disease, early change in quality of life scores of just about anything you measure is predictive of survival. There is a case there that patients know something – particularly the change. There have been a lot of studies in lung cancer, colon cancer, breast cancer, where patients who get better early on in therapy live longer, and patients who get worse early on don’t live as long.”
Cella suggested that analysing how changes in different symptoms and quality of life indicators, measured just before and shortly after progression, correlate with overall survival could help identify markers that can predict survival.
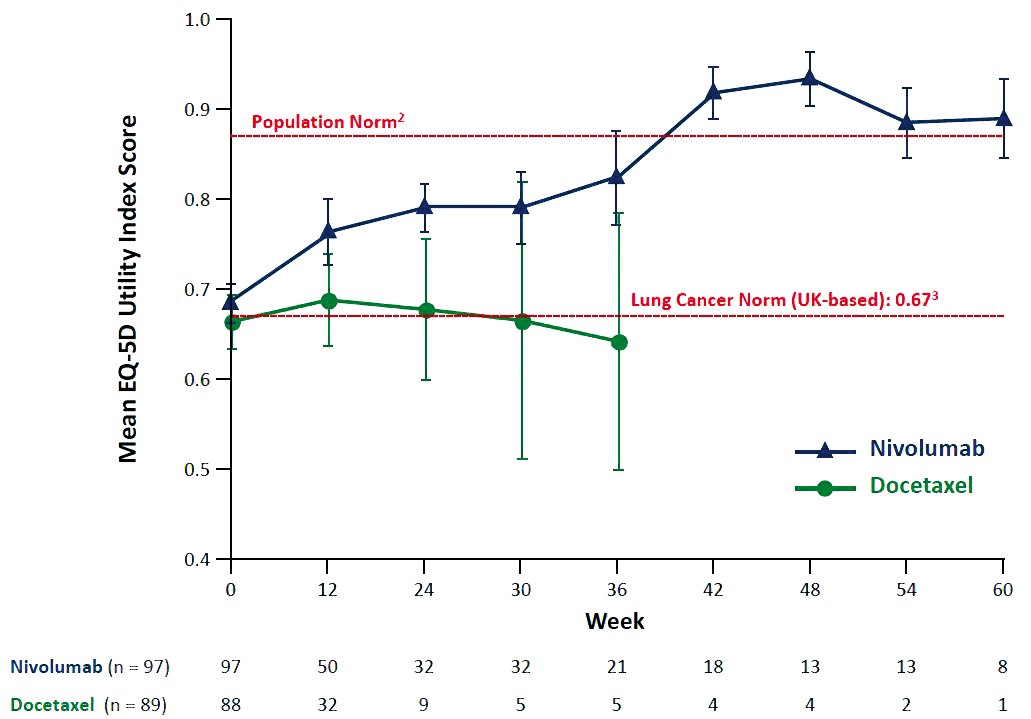
A study of patients treated with nivolumab for advanced non-small-cell lung cancer indicated that, among long-term responders, patient-reported quality of life scores re- turned to population norms at 36–42 weeks (M Reck et al, ECCO-ESMO 2015, Abstract 3011). The study was exploratory, and involved a very small number of patients Courtesy of Martin Reck, LungenClinic Grosshansdorf
Finding the right patients
Immunotherapies currently work well in only a small minority of patients. Finding biomarkers that can predict which patients will benefit is proving surprisingly challenging. Contrary to expectations, the presence of CTLA4 or PD-L1 in tumour cells – both targets for checkpoint inhibitors – does not seem to be a reliable guide to the immune response.
One thing that is clear, according to Genentech’s Mellman, is that, “Everyone who responds to PD-1/PD-L1 therapies are individuals who have some level of pre-existing immunity which you can objectively measure with increasing degrees of accuracy.” Patients who do not respond to immunotherapy either have dysfunctional responses so that T cells never get beyond the periphery of the tumour or “an immune desert” where no T cells at all confront the tumour, he said.
Resolving this issue will be important, not least because the FDA and EMA are becoming increasingly insistent that new cancer therapies be accompanied by validated biomarker tests that indicate in whom they should be used.
Heinz Zwierzina, from the Early Clinical Trials Unit at Innsbruck, who chairs the Cancer Drug Development Forum, argues that it is also an issue of ethics and affordability. “If we treat the ‘wrong’ patients with a drug that does not work and may cause side effects, we may lose time in treating patients with the right drug,” he said, adding that, “Our healthcare systems will be in serious trouble if we cannot define sub-populations of patients.”
The size of the problem can be seen in the striking difference between the cut-off points used in different clinical trials to define ‘PD-L1 positive’, ranging from 1% in a trial of pembrolizumab for melanoma, to 5% for a trial of nivolumab in non-small-cell lung cancer and 50% for pembrolizumab in non-small-cell lung cancer.
Mira Pavlovic-Ganascia, a derma-tologist who coordinated the European Network of Health Technology Agencies (EUnetHTA) for two years, says this discordance makes it impossible for regulators and HTA bodies to compare biomarkers. “It is really frustrating that people use different expression cut-off levels and say it is positive. What are we meant to do with this?”
Francesco Pignatti, who leads cancer drug evaluation for the EMA, says that they cannot ignore the complexities, but have to find a way to be able to compare data. “It is entirely plausible that the relationship does not have a natural threshold telling us there is a single cut off where you can exclude or include patients in the label. It is perhaps a dynamic type of marker across different tumour sites or tumour types. The relationship might be a linear one not a binary cut off.”
Imposing an arbitrary cut-off point, he argued, would be seen as desperately unfair by patients whose PD-L1 marker falls on the wrong side of a dividing line, and could encourage off-label use. On the other hand, leaving it to patients and clinicians to decide could mean that everybody ends up on the drug, regardless of benefit. “How much confidence is there across the European Union about shared decision-making once it ends up on the label?”
EMA to sponsors: Talk early talk often
The European Medicines Agency and the Food and Drug Administration have both adopted a proactive approach to discussing the shape of trials with pharmaceutical companies during the design stage and a willingness to accept ‘breaking news’ evidence after a licence application has been submitted.
The ‘breakthrough’ designation introduced by the FDA in 2012, and the ‘adaptive pathways’ approach piloted by the EMA in 2014, both aim to speed the development and review of new drugs for serious diseases where preliminary data indicate they might be substantially better than what is currently available.
In both cases, the emphasis is on early and frequent access to discussion and advice, and a ‘rolling review’ to help steer the drug (or combination) through the most efficient development pathway.
Pembrolizumab (Keytruda) was the first immunotherapy to be granted breakthrough designation by the FDA in 2013, and was approved for use in patients with advanced melanoma in September 2014. EU approval for the same patient group came in the summer of 2015.
Roger Dansey, head of oncology clinical development at developers Merck (MSD), said that the EMA and its human medicines committee had demonstrated “enormous flexibility and willingness” to accommodate data as it emerged from a trial that was simultaneously assessing clinical benefit and dosage. “We are in a hurry because patients are in a hurry. There is an enormous unmet need. We were very appreciative of that degree of flexibility.”
Bristol-Myers Squibb reported similar flexibility when they applied for marketing authorisation for nivolumab (Opdivo) in advanced squamous non-small-cell lung cancer. Catherine Weil, from BMS Belgium, said, “We submitted the confirmatory study data during the review based on large survival advantage. The boundaries had been clearly crossed and the result was positive. It was very, very collaborative I would say. For squamous non-small-cell cancer patients there has not been a new drug approved in the past 20 years or so. The sense of urgency was really shared.”
The reimbursement hurdle
Marketing approval is one thing – getting the therapy to patients is another. Patrick Hopkinson, director of health economics and outcomes research at BMS UK, said the average time from approval to an agreement for reimbursement for cancer therapies is just over 8 months in France, 9 in Germany, 10 in Italy, almost 11 months in England and just over a year in Spain. In some other EU countries it is even longer.
Francesco De Lorenzo, President of the European Cancer Patient Coalition (ECPC), said that delays in introducing treatment amount to a form of rationing. ECPC is calling for a strengthening of current efforts to coordinate aspects of HTA assessments across member states by introducing a single assessment of relative clinical benefit that would be legally binding across Europe.
Hopkinson argued that HTA bodies need to take a broad view of clinical benefit given the potential for immunotherapy to transform patient outcomes. “We are on the cusp of a revolution in terms of therapies launched or to be launched. Current frameworks for value assessment in many HTA bodies especially for cancer medicines are not built for this class and type of therapy.”
He said the willingness of the FDA and EMA to give accelerated approval on the basis of early evidence was very encouraging, but there could be a mismatch with the criteria HTA bodies required for their assessments. “Time is of the essence if you are a lung cancer patient – 80% of patients are dying within 12 months.”
Mira Pavlovic-Ganascia, former coordinator of EUnetHTA, agreed that some of the mortality data from trials was “amazing”. She argued, however, that HTA bodies want a broader set of data to evaluate “added clinical benefit”, with the main clinical endpoints being how a patient “feels, functions or survives”.
They also need data that can inform national policy on where the treatment fits within the overall treatment pathway and how this differs between patients according to whether their disease is progressing fast or slow.
They are therefore looking for something more meaningful than early data on progression-free survival, and they become “extremely nervous”, for instance, when asked to assess results of a trial using a non-authorised dose of pembrolizumab for NSCLC.
Many countries are, however, looking at various forms of managed entry agreements and risk-sharing arrangements that can allow new medicines to be marketed on the basis of promising early data, while additional data about its value in actual clinical practice are being collected.
A wider horizon
Although it is checkpoint inhibitors causing the excitement today, other forms of immunotherapy could become significant tomorrow, building on new and emerging techniques. One under development is the use of mRNA and peptide-based anti-cancer vaccines based on antigen profiling of the individual tumour. Another uses CAR-modified T cells to attack the tumour. This seems to be highly effective in leukaemias but can also be highly toxic.
One small study (so far unpublished) in February 2016 reported 94% complete remission in patients with acute lymphoblastic leukaemia, along with some very severe side effects. A wave of immunotherapy trials will report at ASCO in Chicago in June.
Meanwhile, patients are increa-singly seeking access to the new wave of immunotherapies. Aleksandra Filipovic, a clinical fellow at Imperial College London, said that the private sector there had started using the latest checkpoint inhibitors within weeks of FDA approval, with patients treated on the NHS now also able to use them through the early access programme.
“The way these drugs have moved forward in the past year and a half – the speed is absolutely staggering. The question we have answered is that the drugs work. Tumours that have been very difficult to treat are now finding their answer in immunotherapies,” she said.
The greatest response rates had been seen in lymphoma, mesothelioma, patients with mismatched repair deficient colorectal cancer and melanoma. Responses of 20% or more have also been reported in patients with advanced NSCLC, heavily pre-treated triple negative breast cancers and gastric cancers.
Filipovic agrees that many questions remain about how to select the most suitable patients, how best to combine therapies, how long to give them for, how to best assess likelihood of response as early as possible and how costs can be met. The main story, however, is that these treatments are offering hope to patients in great need. “It is a space that is exploding at the moment.”
Accelerating progress in immunotherapy: the challenges
Ethics v evidence. Patients don’t want to be put on lengthy trials where early data indicate big differences between trial arms. Adaptive trial designs, and investing resources up front to learn as much as possible through many short exploratory trials with small numbers of patients, will be important.
Endpoints. Finding surrogates that can be measured early on and predict for survival is a priority. Currently there is no agreement on surrogate endpoints. Changes in specific and measurable aspects of how a patient feels is one area for exploration.
Side effects. Monotherapy has shown impressive improvement in quality of life among responders in diseases associated with a very high symptom burden. However, combinations of immunotherapies carry a high risk of serious toxicity. This needs to be overcome, particularly if immunotherapies are to be used long term or as adjuvant therapy.
Personalisation. Finding biomarkers that predict who will benefit is turning out to be very difficult. More basic understanding is needed of exactly how immunotherapies work.
Reaching patients. Working with HTA on managed entry and risk-sharing agreements to secure early access to new treatments while gathering robust information on value remains a challenge.






Leave a Reply