Delaying treatment for a curable prostate cancer is an increasingly popular option among men with low risk tumours. Simon Crompton looks at efforts to learn more about who benefits and how to avoid delaying too long.
Active surveillance is an increasingly popular observational approach to managing low and very low risk prostate cancer. The American CaPSURE prostate cancer database indicates that its use as an initial management strategy for men in these risk categories quadrupled from one in ten to nearly four in ten between 2009 and 2013. The approach, which gives men with localised prostate cancer the opportunity to avoid or delay radical treatments, is now regarded as quite different to watchful waiting.
While the aim of treatment administered after watchful waiting is to control the cancer, treatment after active surveillance has the aim of cure.
There are other differences. Active surveillance involves a schedule of assessments and tests such as PSA (prostate specific androgen) tests, biopsies and clinical examination. Watchful waiting, which will more commonly apply to men with a life expectancy of less than 10 years, involves clinic visits and PSA testing.
The field has specialised rapidly in the past 15 years, with the European School of Oncology taking a lead in extending knowledge – organising three conferences gathering expertise in urology, radiology, biology, psychology and public health. The growth in interest has coincided with mounting concern about overtreatment in prostate cancer.
And now there is new evidence of how effective active surveillance can be. This September, the New England Journal of Medicine published the first results from a major trial that compared treatment outcomes in 1,643 men with localised prostate cancer (doi: 10.1056/NEJMoa1606220).
They indicated that men survive just as long with active surveillance as with radical prostatectomy surgery and radiotherapy.
The ProtecT (prostate cancer testing and treatment) trial, led by the universities of Oxford and Bristol and involving nine centres, followed patients whose localised cancer had been detected after PSA tests.
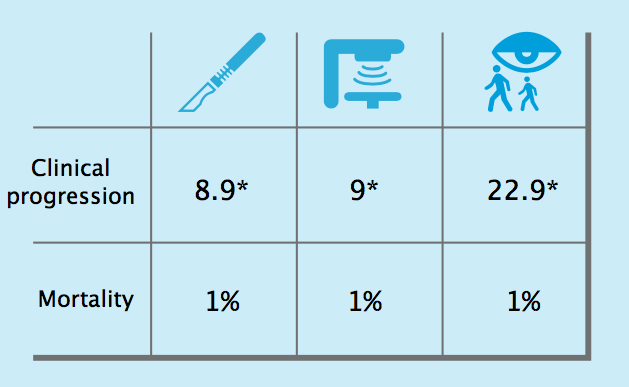
It found that 10-year prostate-cancer-specific survival was 99% whichever treatment approach was assigned.
However, after six years, twice as many men who had surgery experienced continence and sexual problems compared to those who had active monitoring and radiotherapy. And radiotherapy caused more bowel problems than surgery or active monitoring.
When treatment does more harm than good
For many men, prostatectomy, radiotherapy and brachytherapy are likely to produce effects far worse than their cancer ever would. Studies indicate that around two men in every ten have long-term urinary incontinence following radical prostatectomy, and between three and seven men in every ten who undergo radical prostatectomy or external beam radiation therapy will develop impotence after treatment.
In contrast, recent studies of men with low risk prostate cancer indicate that fewer than one in ten of them on active surveillance programmes have died of the disease after 15 years.
There is excitement among much of the prostate cancer community about advancing this field, and in so doing offering many men the prospect of a long and fulfilled life free of treatment side effects. Yet it is an emerging art, with consensus and evidence on eligibility and the best monitoring approaches elusive.
The thinking behind active surveillance
Unlike many other malignancies, prostate cancer often grows slowly and consistently over time, sometimes producing no symptoms at all. Autopsy studies have shown that as many as eight in ten men in the 60- to 80-year age group who die of other causes have cancer in their prostates without even knowing it.
Epidemiological studies have also indicated that many men have indolent and asymptomatic prostate cancer that should not require treatment.
And yet there always remains the possibility that any diagnosed prostate cancer will grow rapidly, metastasise and become lethal.
Active surveillance is an approach that attempts to straddle these difficult poles. It is based on the assumptions that:
- All prostate cancer treatments, including those directed at minimal disease, are often associated with significant side effects and costs.
- It is possible to distinguish indolent prostate cancers from those that will lead to symp-toms, metastases and death.
- After biopsy, active surveillance patients can be reclassified as being at higher risk of disease progression, and receive treat-ment, without reducing the chance of cure.
- For some people, the burden of living with disease is less than living with the effects of unnecessary treatment.
For clinicians, active surveillance involves three key decision-making areas:
- Is the patient at low or very low risk of progression?
- How will the patient be moni-tored during active surveillance?
- How will it be decided whether treatment should start?
Does it work?
Since active surveillance is a relatively new approach, conclusive evidence about its value is still scarce. Large randomised trials are underway, most significantly the ProtecT trial.
The first results from the ProtecT study, indicating that surgery, radiotherapy and active surveillance all result in a similar mortality rate of around 1% after ten years, lend strong support to active surveillance as a treatment approach.
Results of the ProtecT trial
Ten-year outcomes from the ProtecT trial, which randomised men with low risk localised prostate cancer to surgery, radiotherapy or active sur- veillance, showed that, while clinical progression was more likely in men managed by active surveillance (22.9* vs around 9* for surgery and radio- therapy), the chances of dying of prostate cancer were equally low for all three management options, at 1%.*Per 1000/person years
Source: FC Hamdy et al (2016) NEJM, published online 14 September 2016, doi:10.1056/NEJMoa1606220
However, after ten years the study did find evidence of more cancer progression and metastases in men assigned to active surveillance than those assigned to surgery and radiotherapy.
A New England Journal of Medicine editorial accompanying the ProtecT results said this meant that, if a man wanted to avoid metastatic prostate cancer, “monitoring should be considered only if he has life-shortening coexisting disease such that his life expectancy is less than the 10-year median follow-up of the current study.”
Until the ProtecT results, evidence has mainly come from observational studies. These have consistently found a low rate of progression to metastatic disease or death in patients on active surveillance. The majority of patients in the studies do not go on to require treatment.
In a prospective study from Toronto looking at 993 men managed with active surveillance since 1995, there was a 95% metastasis-free survival rate after ten years (most were low risk but 20% were classed as intermediate risk). At Johns Hopkins University, a study of 1,298 men on active surveillance revealed a prostate cancer mortality of just 0.4% after 15 years.
However, differing survival rates are partly a reflection of which patients are selected for active surveillance: mortality is likely to be small when only the lowest risk groups are admitted to this approach. Inclusion criteria vary from centre to centre.
Evidence on quality of life is encouraging. A new analysis of data from four military centres participating in the Center for Prostate Disease Research Multicenter National Database found that, apart from a slight difference in bowel function, health-related quality of life outcomes for patients on active surveillance were no different from those in men without prostate cancer during the three years of follow up.
Balancing risks and benefits
Active surveillance performs a balancing act between reducing over-treatment and reducing the risk of death. And although the potential benefits are great, there are risks: particularly that the window of curability is missed and that switching to curative treatment comes too late.
As Lionne Venderbos from Erasmus University pointed out at ESO’s recent active surveillance conference in Milan, this has potential legal and ethical ramifications – so patient involvement in decision making is absolutely essential.
How do you minimise risk while also bringing benefits to the maximum number of men? The answer lies in selecting the right people for active surveillance, but the criteria used are still a matter of debate.
Selecting the right tumours for active surveillance
The difficulty of differentiating low risk ‘pussy cat’ indolent tumours from the high risk ‘tiger’ tumours has always run central in prostate cancer management decisions. There are no definitive indicators of low risk tumours, so there are no definitive indicators for the tumours most suitable for active surveillance.
This means that different centres and studies have different criteria. However, according to Laurence Klotz, Professor of Surgery at the University of Toronto, all inclusion criteria will have the following in common:
- the cancer will be at an early clinical stage (extent)
- there will be a relatively low serum PSA reading (volume)
- the tumour’s Gleason score will indicate it is well, or moderately, differentiated (grade or clinical behaviour).
Other clinical measures that are often used to determine whether the tumour is low risk include:
- the number/percentage of positive cores on original biopsy
- the extent of tumour involvement within a biopsy core
- the PSA density
- the PSA kinetics.
Selecting the right people for active surveillance
The likelihood of cancer causing death depends not only on the extent and aggressiveness of the tumour but on patient characteristics, particularly age, co-morbidities and life expectancy. The ethnicity of a patient may also be a consideration. In African Americans, for example, prostate cancer has a significantly earlier age of onset, higher PSA levels, worse Gleason scores, and more advanced stage at presentation. Studies indicate that this population has a higher rate of unfavourable findings at prostatectomy than other ethnic groups.
According to Athene Lane, Reader in Trials Research at the University of Bristol, selection of patients for active surveillance may also be enhanced by knowledge of their psychological status at diagnosis. Around two men in ten move off active surveillance without evidence of clinical progression, and the reasons may be psychological.
An increasingly popular option
Data from the US show a rapid rise in men opting for active surveillance in preference to surgery or radiotherapy, between 2009 and 2013, from 1 in 10 to 4 in 10
Source: CaPSURE national registry of men with prostate cancer diagnosed at 45 urology practices across the United States, cited by MR Cooperberg and PR Carroll (2015) JAMA: 314:80–2
Prospective active surveillance patients need good decision-making aids, according to Lara Bellardita, Clinical Health Psychology Consul-tant at the IRCCS Istituto Nazionale dei Tumori Foundation, Milan, Italy. “Active surveillance involves a complex decision-making process and it is highly influenced by physicians’ preferences and ability to engage the patient in shared decision making” she says.
Debate about eligibility criteria
In the absence of long-term studies characterising the type of disease and person suitable for active surveillance, researchers are trying to find new predictors of disease progression to support risk-based selection. Existing prediction models help but, as Ewout Steyerberg from the Centre for Medical Decision Making at Rotterdam’s Erasmus University has pointed out, much stronger predictors are needed to separate low risk from high risk patients.
At ESO’s recent active surveillance conference in Milan, participants discussed the merits and difficulties of expanding active surveillance beyond people with low and very low risk cancers, where it is agreed that the approach works well.
The UK NICE Protocol for Active Surveillance
National Institute for Health and Care Excellence (2014) Prostate Cancer: Protocol for Active Surveillance. CG175. National Institute for Health and Care Excellence, London
Laurence Klotz provided details from Toronto illustrating the challenge. In a study of 980 patients, a highly restrictive approach selecting only very low risk patients resulted in a 15-year mortality of 0.5%. An inclusive approach, including all low risk and selected intermediate risk patients, resulted in a 15-year mortality of 5%. But excluding Gleason 7 patients from this group would have brought down the figure to 2%.
Karim Touijer, Attending Surgeon at Memorial Sloan-Kettering Cancer Center, said that if active surveillance were to be used for higher risk cancers, with high volume and Gleason readings of 3+4, there was a need for better prognostication. Klotz, Touijer and many other prostate cancer specialists believe that the use of MRI scans, fusion-guided biopsy and biomarkers all offer opportunities to refine patient selection (see developments in active surveillance, below).
Surveillance strategy
Despite a multitude of guidelines, there is no consensus on the best strategy for managing prostate cancer with active surveillance.
The main priority in all strategies is to detect evidence of reclassification or progression. This regular monitoring is likely to include:
- serum PSA testing
- digital rectal examination
- repeat prostate biopsy.
There are as yet no clinical studies that define the best testing intervals and criteria to trigger active intervention.
According to Leonard Bokhorst from the Department of Urology at Erasmus University, the Netherlands, the goal of follow-up testing is threefold: to filter out incorrect selection (reclassification); to filter out tumours that progress; and to do so with the minimum amount of harm to the patient. So the frequency of testing should be tailored to the individual – based not only on the risks and benefits but also the demands and discomfort caused by procedures such as biopsy.
What should trigger reclassification of a tumour during surveillance and prompt the start of treatment? Cohort studies show large variations in criteria, says Antti Rannikko, senior consultant urologist at Helsinki University Hospital, Finland. “Most rely on repeat biopsies to monitor grade of the disease,” he says. “The volume of the disease is generally monitored with biopsy-based surrogates, such as number of positive biopsies, and PSA-based surrogates, such as free PSA and PSA doubling time.”
According to Peter Carroll, Professor and Chair at the Department of Urology, University of California, the growing global demand for active surveillance means there is an urgent need to refine surveillance protocols. He believes the use of MRI imaging will play an important part in this, particularly in decisions on when to upgrade tumours and begin intervention.
Current guidelines on active surveillance
There are some guidelines that attempt to identify both the patient groups for whom active surveillance is an appropriate option and the surveillance strategy itself. These include:
- The American Urological Association (AUA) Guideline for the Management of Clinically Localized Prostate Cancer (US)
- The European Association of Urology (EAU) Guidelines on Prostate Cancer
- The Cancer Care Ontario Guideline, endorsed by the American Society of Clinical Oncology (Canada)
- National Comprehensive Cancer Network Guidelines for Prostate Cancer (US)
- The National Institute for Health and Care Excellence (NICE) Guideline on Prostate Cancer, including a protocol for active surveillance (UK).
A summary of the protocols published by NICE (UK) and Ontario Care (Canada) are shown as examples, in the boxes on this and the facing page.
The GAP 3 project
Definitive answers about how to select men for active surveillance and then successfully monitor them are likely to come from large studies analysing existing data from men with prostate cancer – and in particular a major study funded by the Movember Foundation, known as the Global Action Plan 3 project, or GAP3. Movember supports five GAP projects, but GAP3 specifically addresses selection for active surveillance.
It aims to create global consensus through studying the cases of 14,000 men across 19 institutions worldwide. This is the largest prostate cancer active surveillance database, comprising the majority of the world’s active surveillance patient data.
Two years into the project, all the data from participating centres has been uploaded into a central database. Each patient’s clinical history and data from biospecimens, imaging and biomarkers is being analysed.
This analysis will feed into a simultaneous expert review of all current active surveillance guidelines available around the world, leading to a new consensus guideline setting out which patients are suitable for active surveillance, and which are the most effective ways of monitoring them.
The end result will be a web-based platform, based on the guidelines and using risk-based modelling derived from the new analysis, to help clinicians decide which patients are suitable for active surveillance.
Perhaps just as importantly, says Sophie Bruinsma, the researcher from Erasmus Medical Centre who is coordinating the project, it will also provide some reassurance to men that they have made the most sensible, risk-based decision about their disease.
Developments in active surveillance: MRI
With increasing recognition that PSA testing is a blunt tool in both diagnosis and monitoring – which has also led to a skyrocketing of prostate cancer diagnoses in the Western world – more tests are being added to the armoury.
Imaging techniques such as multiparametric MRI and the use of new biomarkers hold particular potential in both enhancing diagnostic accuracy and monitoring prostate cancer, though as Laurence Klotz has pointed out, both are “promising but imperfect”.
Multiparametric MRI scanning has four potential roles in active surveillance.
First, at diagnosis, it provides information on various aspects of tissue make-up including cell density. Second, it can be helpful in guiding confirmatory biopsies after a negative first biopsy but rising PSA levels. Third, it has a role in guiding repeat biopsies. Finally, during follow up, it can be used to assess any change in the cancer and trigger repeat biopsies.
However, debate continues about the exact value of multiparametric MRI in active surveillance. It was a key area of discussion at ESO’s recent conference in Milan. Jochen Walz, Head of the Department of Urology at Marseille’s Institut Paoli-Calmettes Cancer Centre, emphasised that only high-quality imaging can improve the management of active surveillance patients.
Multiparametric MRI is helpful to guide biopsy, but its use in monitoring patients on active surveillance needs to be defined, said Caroline Moore, Senior Lecturer at the Division of Surgical and Interventional Science, University College London.
Peter Caroll and Michael Leapman described how the use of multiparametric MRI had had an impact in the active surveillance of men with low to intermediate risk prostate cancer at the University of California San Francisco.
The Ontario Cancer Care Protocol for Active Surveillance
□ Active surveillance is recommended for most patients with low-risk (Gleason score of 6 or less) localised prostate cancer.
□ Some patients with low-volume, intermediate-risk (Gleason 3 + 4 = 7) prostate cancer may be offered active surveillance.
□ Factors including age, prostate cancer volume, patient preference, and ethnicity should all be taken into account in decisions.
□ Surveillance protocols should include PSA testing, digital rectal examinations, and serial prostate biopsies.
□ Additional scanning and genomic tests may have a role in patients with unclear findings.
□ Patients who are reclassified to a higher risk category (Gleason score of 7 or more), or who have significant increases in tumour volume, should be offered active therapy.The full Cancer Care Ontario guideline, as endorsed by the American Society of Clinical Oncology, can be found at http://jco.ascopubs.org/content/34/18/2182. full
In a study of 1,480 men, they concluded that it was a useful diagnostic and staging modality for men with newly diagnosed prostate cancer, particularly when used in conjunction with ultrasound to guide biopsy (MRI and ultrasound fusion guided biopsy). And during surveillance, fusion guided biopsy improved detection of clinically significant prostate cancer in a proportion of men.
However, the benefits of serial imaging as a means of surveillance are still unclear and under-researched.
Caroline Moore pointed to methodological difficulties involved in studying serial imaging. She said that “radiological progression” was hard to measure over time, because of changes in scan quality, physical changes in the patient and natural variations in measurements.
“We need a multi-institutional analysis using an agreed minimal data set on repeat multiparametric MRI to answer questions of natural variation and tumour size kinetics,” she said.
Developments in active surveillance: molecular markers
The use of new chemical and genetic markers to monitor disease and help predict its course is now being seen as another tool with potential for active surveillance.
According to Bruce Trock, Professor of urology, epidemiology, oncology and environmental health sciences at the Johns Hopkins School of Medicine, in Baltimore, measuring circulating biomarkers may help determine eligibility for active surveillance, providing a basis for reclassifying tumours (and possibly beginning treatment) and providing prognostic clues.
Some biomarkers may capture the heterogeneity of tumours better than biopsy. However, few have been properly evaluated.
Both blood and urine biomarkers hold potential, for example:
- Blood: various characteristics of PSA (kinetics and isoforms)
- Urine: PCA3 and TMPRSS2-ERG.
Examining these sorts of molecular markers may have a particular role in increasing clinicians’ confidence that the right patients are being selected for active surveillance, according to Sigrid Carlsson, Assistant Attending Epidemiologist at Memorial Sloan Kettering Cancer Center in New York.
Active Surveillance Conference
Every two years the European School of Oncology hosts an expert conference gathering latest evidence on active surveillance and the technologies that may improve selection and monitoring of low risk prostate cancer patients. It is held in collaboration with the European Association of Urology and the patient advocacy group Europa Uomo, and attracts urologists, oncologists, radiologists, psychologists and public health experts from around the world.
The 4th Conference on Active Surveillance for Low Risk Prostate Cancer will be in 2018.
More information is available on the prostate cancer programme page at www.eso.net.
Current prostate cancer guidelines from the European Association of Urology (2015) state that, while biological markers and genomic analysis are promising, “further study data will be needed before such markers can be used in standard clinical practice.”
However, Antti Ranniko, a urolo-gist from the University of Helsinki, is optimistic that both molecular testing and multiparametric MRI scanning will hold increasing importance in active surveillance.
“Initial reports of multiparametric MRI’s negative predictive value of close to 100% for clinically significant cancer seem promising for active surveillance,” he said in Milan. “Also, initial reports on genetic tests to predict cancer outcome are noteworthy, and it is tempting to speculate that the future triggers for reclassification will largely rely on multiparametric MRI and genetic biomarkers.”
The patient perspective
How do patients respond to active surveillance? Recent reviews of quality of life under active surveillance indicate that overall quality of life is good in the first few years of surveillance, with low levels of anxiety and depression.
“I was just thankful that, you know, that it is being monitored and…if it does start going a bit wild then I’m obviously in the right place to have it sorted…”
“They do just ask how do you feel and whether it’s giving you any trouble. When I go to [hospital] if I am a little worried and I do talk to them, they put me at my ease.”“ I woul
d put it at the back of [my] mind but so
me days it’d come to the front and I do start thinking then.”“You think you can handle it but it’s always there niggling away in your mind.”
“Just a routine now, don’t think much to it really, so that’s the way it is… I’m fine with it. I don’t fret on it, I’m not anxious about it. I just wait to see what happens.”
Quotes are taken from interviews with 22 men with early prostate cancers being managed with active surveillance, as part of a qualitative study within the ProtecT trial. The interviews were carried out face-to-face or by telephone
A systematic review led by Lara Bellardita from the IRCCS Istituto Nazionale dei Tumori Foundation, Milan, found that quality of life scores were equal to, or better than, those for patients who had undergone radical treatment.
Another review of both active surveillance and watchful waiting evidence, led by Gregory Carter from the School of Medicine and Public Health at the University of Newcastle, Australia, concluded that decisional conflict was low and decisional satisfaction high.
New results from the ProtecT trial on patient reported outcomes indicate that, although active surveillance patients report fewer adverse effects from treatment than those undergoing surgery or radiotherapy, over six years the health related quality of life scores (including anxiety and depression) for all three groups of patients were similar.
Athene Lane, from the University of Bristol, says that interviews with patients being managed with active surveillance as part of the trial have showed that they particularly benefit from peer and partner support, as well as from positive experiences with health professionals and recognition of their uncertainty and emotional responses.
Some of the men interviewed indicated that they trust clinicians to monitor their disease closely, and that initial worries preceding PSA tests reduce with time.
One respondent who had been on active surveillance for five years said: “It’s just a routine now. I don’t think much to it really, it’s the way it is… I just wait to see what happens.”






Leave a Reply