When your patient is pregnant, uncertainty about the impact different therapies may have on the child makes it hard to discuss treatment options. A study of children exposed to cancer treatments before birth is now starting to provide some welcome evidence.
ESO presents weekly e-grandrounds which offer participants the opportunity to discuss a range of cutting-edge issues, from controversial areas and the latest scientific developments to challenging clinical cases, with leading European experts in the field. One of these is selected for publication in each issue of Cancer World.
In this issue, Kristel van Calsteren, from the Leuven University Hospital, Belgium, reviews registry data on the impact that treating cancer in pregnant women has on the children they give birth to. The presentation was summarised and edited by Susan Mayor
The recorded version of this and other e-grandrounds, is available at www.e-eso.net
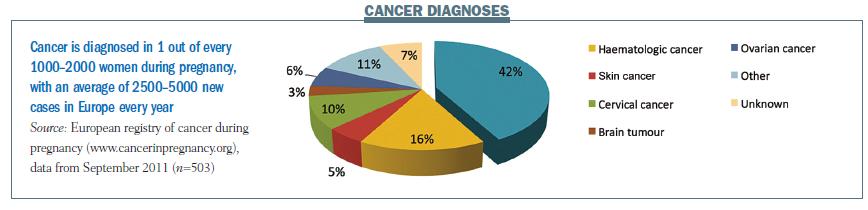
Cancer is diagnosed in one in every 1000–2000 pregnancies, with 2500–5000 new cases in Europe each year. The European registry had registered 500 patients at the last analysis in September 2011. Nearly half of the women included had breast cancer (42% of all patients), while 16% had haematological malignancies, 5% had skin cancer and 10% had cervical cancer.
Pregnancy can be considered in three phases when considering the potential risks of chemotherapy to the developing foetus.
The first stage covers the first 10 days after conception – the implantation phase. A toxic event at this stage may cause some of the pluripotent stem cells that make up the early foetus to die. The embryo will develop normally if most cells survive but the pregnancy will end in miscarriage if a significant number of cells die.
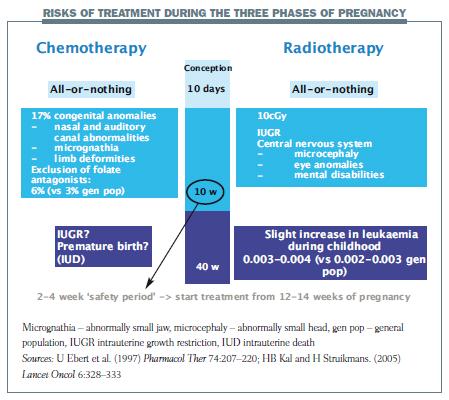
The most dangerous period of pregnancy for cancer treatment is the first 10 weeks, when organs are being formed and developed. Chemotherapy in this period was associated with congenital anomalies in 17% of children. When folate antagonists were excluded, only around 6% of children were affected (Pharmacol Ther 1997, 74:207–220).
 Later in pregnancy, from 10 to 40 weeks, there is no increased risk of congenital malformations. However, case studies report an increased number of babies with growth restriction, pre-term deliveries and even intrauterine deaths.
Later in pregnancy, from 10 to 40 weeks, there is no increased risk of congenital malformations. However, case studies report an increased number of babies with growth restriction, pre-term deliveries and even intrauterine deaths.
Radiotherapy has a similar ‘all-or-nothing’ impact as chemotherapy at the beginning of pregnancy. Later in pregnancy the maximal safe limit for foetal exposure is 5–10 cGy. We know that if foetal exposure is more than 10 cGy you will see foetal growth restriction, and also important problems in the development of the central nervous system. In the long term, a slight increase in leukaemia has been described during childhood.
 International follow-up study
International follow-up study
An international, collaborative long-term study is following up children after prenatal exposure to chemotherapy in Belgium, the Netherlands and the Czech Republic. It is examining the long-term effects of prenatal exposure to chemotherapy, including the obstetric and neonatal outcome, general health, development, neurological outcome, and cardiac outcome for each child.
The study was initiated in 2005 by Frédéric Amant and included 114 children born after prenatal exposure to chemotherapy (31 children born before 2005 and 83 after). Seventy children (32 girls and 38 boys) were included in the long-term follow-up study after excluding children younger than 18 months, those with incomplete medical files, one child who died due to sudden infant death syndrome, and those who were lost to follow-up or who refused to participate (mainly because they did not want to return to hospital). The median follow-up period was 22 months.
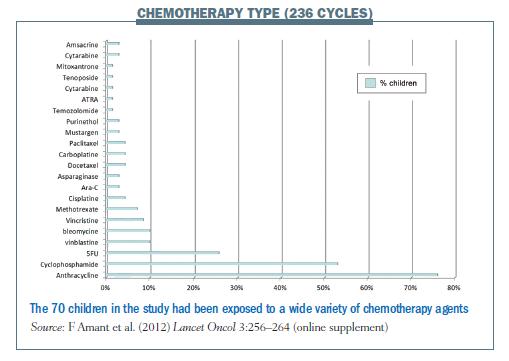
The median gestational age at cancer diagnosis was 18.1 weeks; breast cancer accounted for 51.5% of these diagnoses and haematological cancers accounted for a further 26.5%. Thirty-four of the women (50%) received chemotherapy during pregnancy. More than one-third (39.7%) had surgery plus chemotherapy during pregnancy, one woman had chemotherapy plus radiotherapy, and six had surgery, chemotherapy and radiotherapy. Altogether, the children had been exposed to a total of 236 chemotherapy cycles (see figure below for chemotherapy type), with the most commonly administered drugs being anthracyclines, cyclophosphamide and fluorouracil. These are the chemotherapy drugs most commonly used to treat breast cancer, which accounted for half of cases.
 The children were examined at birth and at 18 months, and were then followed up every three years. The tests administered included: cardiac examination with an ECG and an echocardiography; general health assessment, which consisted of a paediatric examination and questionnaire completed by parents; neurological examination, which consisted of a clinical examination at birth and a Bayley test at the age of 18 months. From the age of six years, a one-off audiometry test and an age-adapted neuropsychological test battery measuring intelligence, attention and behaviour was performed. Intelligence was tested at the age of six years using the WPPSI-R test, and the WISC-III or WAIS-III in older children. Attention was assessed using the Test for Everyday Attention for Children (Tea-Ch); memory was checked using verbal and non-verbal tests in the Children’s Memory Scale and the Auditory Verbal Learning Test. Behaviour was tested with the Child Behaviour Checklist, which was completed by parents.
The children were examined at birth and at 18 months, and were then followed up every three years. The tests administered included: cardiac examination with an ECG and an echocardiography; general health assessment, which consisted of a paediatric examination and questionnaire completed by parents; neurological examination, which consisted of a clinical examination at birth and a Bayley test at the age of 18 months. From the age of six years, a one-off audiometry test and an age-adapted neuropsychological test battery measuring intelligence, attention and behaviour was performed. Intelligence was tested at the age of six years using the WPPSI-R test, and the WISC-III or WAIS-III in older children. Attention was assessed using the Test for Everyday Attention for Children (Tea-Ch); memory was checked using verbal and non-verbal tests in the Children’s Memory Scale and the Auditory Verbal Learning Test. Behaviour was tested with the Child Behaviour Checklist, which was completed by parents.
Impact on the children
Birth weight
The median gestational age at birth was 35.7 weeks (range 28.3–41.0 weeks) and the median birth weight was 2612g (range 720–3970 g). Approximately 20% of children (14 out of 70) had restricted growth, which resulted in a birth weight below the 10th percentile for their gestational age.
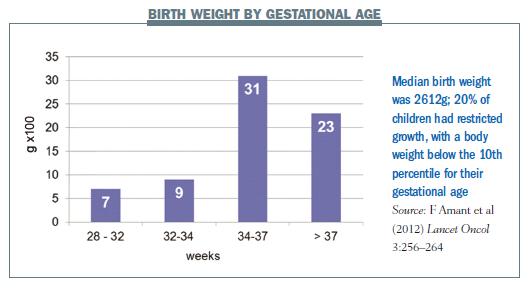 Results for biometry tests, including weight, height and head circumference, for both males and females, showed all the measurements were within the normal ranges.
Results for biometry tests, including weight, height and head circumference, for both males and females, showed all the measurements were within the normal ranges.
With regard to the neurological development, there were two outliers, which related to twins whose mother had been treated for acute leukaemia. At 32 weeks gestation she had pre-term rupture of the membranes and she delivered at 33 weeks. Her son had a birth weight of 1630 g, which was on the second percentile, and her daughter had a birth weight of 1400 g.
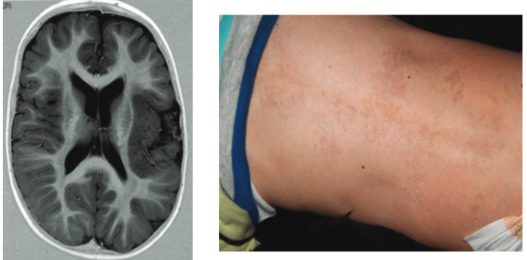
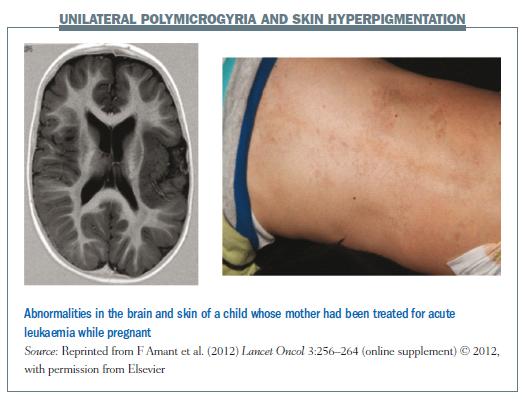 The parents noticed developmental problems with the boy at the age of one year and he has subsequently been diagnosed with autism, and with mental and motor disabilities, which is linked to unilateral polymicrogyria – one side of his brain has an unusually high number of small ridges or folds (see figure below). He also has some dysmorphic characteristics, including hyperthyroidism, low-set ears and areas of hyperpigmentation on the skin, which according to different genetics specialists we spoke with is suggestive of a syndromal problem. The girl initially developed quite well, but at the age of five years had some problems at school. She is now getting some individual support, but is still attending a normal school.
The parents noticed developmental problems with the boy at the age of one year and he has subsequently been diagnosed with autism, and with mental and motor disabilities, which is linked to unilateral polymicrogyria – one side of his brain has an unusually high number of small ridges or folds (see figure below). He also has some dysmorphic characteristics, including hyperthyroidism, low-set ears and areas of hyperpigmentation on the skin, which according to different genetics specialists we spoke with is suggestive of a syndromal problem. The girl initially developed quite well, but at the age of five years had some problems at school. She is now getting some individual support, but is still attending a normal school.
Neonatal neurological examination
Neurological findings were normal in 91% of the children. Five children had transient hypotonia (reduced muscle strength), one child had benign sleep myoclonus (involuntary twitching) and one child, who was born at a gestational age of 28 weeks, had contracture of the right elbow.
Congenital malformations
In the group receiving chemotherapy, one child had hip subluxation, one had a haemangioma and one had a hollow chest (pectus excavatum). Other malformations seen in children exposed to combination therapy included minor malformations of the fingers or the ears, and one child had rectal atresia. Looking at these numbers across the whole group, these are normal incidences that we could expect in the population as a whole.
General health problems
The most important general health problems reported by the parents were those frequently seen in the general population, such as upper airway infections, visual impairment, tonsillectomy, ear tube surgery and speech therapy.
Audiometry
Audiometry results are available for 21 children, as testing is only performed from the age of six years. Eighteen children had normal hearing function and three children had hearing loss. Two of the children with hearing loss are the twins mentioned previously, and the third is a girl with hearing loss diagnosed at the age of six years. Her CT scan showed retracted tympanic membrane. This child also has a history of relapsing ear infections.
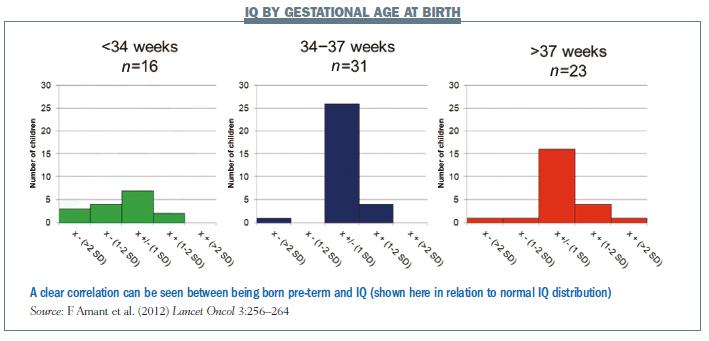
Cognitive function
Results of cognitive function – the IQ and Bayley tests – showed a normal distribution. However, plotting IQ against gestational age at birth showed that children with lower IQ scores were mainly those born pre-term (see figure below). On linear regression there was an increase of 2.5 IQ points for each week increase in pregnancy duration (P=0.0003). This is very important to consider if you decide to induce labour or to perform a Caesarean section.
Results of the other neurological tests for behaviour, memory and attention were nicely in the normal range.
 Cardiac structure and function
Cardiac structure and function
There were no congenital heart malformations. ECGs were all normal, as were measurements of heart diameters. The children also all had normal systolic and diastolic heart functions.
Comparing ECG data for children who were exposed to anthracyclines in utero with matched controls of the same age and gender not exposed to any drug before birth showed results were within expected ranges. Shortening fractions and ejection fractions were significantly lower in the group exposed to anthracyclines in utero, but were still within the normal ranges for their ages, so while the differences were statistically significant they were not clinically significant.
Clinical implications
Results from the study so far are reassuring, although there are some limitations and we should keep the study going for a longer period and include more children. However, at this stage the low rates of complications in babies born to women treated for cancer during pregnancy should lead to less delay in maternal treatment, fewer terminations of pregnancy and fewer iatrogenic premature deliveries.
The children in the study were exposed not only to chemotherapy, but also to other drugs including supportive drugs such as anti-emetics, including metoclopramide and ondan-setron (see table below). Aprepitant is not well studied but animal data suggest low risk, so it can be used in a pregnant woman if you think she really needs it. For corticosteroids, we know that after the first trimester you can use prednisolone and hydrocortisone. We prefer not to give betamethasone or dexamethasone. The difference is that prednisolone and hydrocortisone are metabolised in the placenta, which protects the baby against high-dose cortisol exposures. Betamethasone should therefore only be given for lung maturation; prednisolone or hydrocortisone is preferred for other indications such as anti-emetic effects.
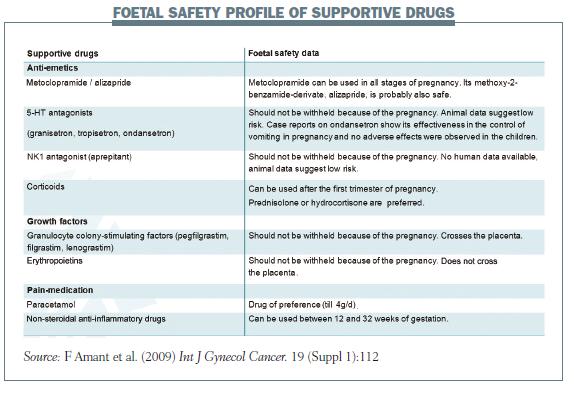 Growth factors are not well studied, but can be given during pregnancy if required. Paracetamol can be used for pain relief. Non-steroidal anti-inflammatory drugs (NSAIDs) can be used between 12 and 30 weeks, but after 30 weeks there is an increased risk of pre-term closure of the ductus arteriosus, which can be fatal for the foetus.
Growth factors are not well studied, but can be given during pregnancy if required. Paracetamol can be used for pain relief. Non-steroidal anti-inflammatory drugs (NSAIDs) can be used between 12 and 30 weeks, but after 30 weeks there is an increased risk of pre-term closure of the ductus arteriosus, which can be fatal for the foetus.
Apart from drug effects, exposure to radiation – diagnostic or therapeutic – can affect the developing foetus. One option, which we used in a patient irradiated for tongue carcinoma while she was pregnant, is to make a phantom to assess the amount of foetal radiation exposure, which can be used to make a decision on whether or not to treat with radiotherapy.
A patient resource
We have developed a website on the treatment of cancer in pregnant women (uzleuven.be/kanker-en-zwangerschap), with a link to the European online registry, which is available in Dutch and English. One important feature is a forum where patients can get in touch with one another. The European Society of Gynaecological Oncology also has a taskforce on cancer treatment during pregnancy (www.esgo.org/Pages/default.aspx) and people interested in this subject are always welcome to participate in this work.
In summary
Chemotherapy can be given with generally low risk to the developing foetus after 12–14 weeks of pregnancy. Results of a follow-up study show the general health, behaviour, hearing and growth in children exposed to chemotherapy during foetal development are similar to the general population. Most of the children in our study had neurological development (intelligence, attention, memory) that was considered adequate for their age and normal cardiac function. However, prematurity was common in this group of patients and was associated with impairment in cognitive development.
The data in this presentation come mainly from an ongoing long-term follow-up study van Calsteren is involved with, of children who have been exposed to chemotherapy before birth (Lancet Oncol 2012, 3:256–264).
Fedro Peccatori (FP), from the European Institute of Oncology, in Milan, Italy, hosted a Q&A session with Kristel van Calsteren (KC).
Q: What about dental problems in children? Dental genes develop during pregnancy so you may expect some problems if there was a specific toxicity on dental genes. Have your babies been seen by a dentist or a specialist in orthodontics?
KC: We put this question to the parents in the general health questionnaire and up until now there have been no specific notes of dental problems in these children. In the follow-up protocol there is currently no central dental examination included, but the children we have seen do not have any more dental problems than other children.
Q: None of your children had hearing problems apart from two children who had slight hearing impairment. Can you comment on this? Why did you look at hearing problems?
KC: We looked at this because we were concerned that there would be neurotoxicity, particularly in the children born to women receiving platinum-based chemotherapy, . This could be expressed in ototoxicity and hearing problems, because this is what we see in patients exposed to these types of drugs. In our registry we recently had one young child with hearing problems after being exposed to combination treatment with a lot of different chemotherapy drugs. However, all the additional children who were examined after the publication of results from the first 70 children had normal hearing function.
Q: What kind of dosages did you use in your patients? Did you adapt the dosage to the distribution volume? Did you use special formulas, such as those in use in oncology for cachectic or obese patients? What was the protocol?
KC: We used the standard chemotherapy regimens we use for non-pregnant patients, and we corrected every cycle for body surface area. For the pregnant patients in the three-weekly regimens when there is a weight gain, the dosage will increase every cycle. But we did not adapt for the pregnancy, only for body weight, as we do in non-pregnant patients. FP: I think this is reasonable even if we have higher clearance, and probably a low protein balance, if things change during pregnancy. But I think that we should stick to what we are used to doing in non-pregnant patients.
Q: Are you planning any co-operation with any other international registries, because this is such a rare situation, with only one in one thousand pregnancies being complicated by malignant diseases?
KC: For the moment we have contact with the Canadian group at the Hospital for Sick Children in Toronto; they also have a large data set. We are not yet collaborating in publishing data together, but I do hope that in future we can put some data sets together to improve the information we can give to the parents.



Leave a Reply