TKIs are involved in treating an increasing number of cancer indications. Doctors need to be aware of the range of potentially serious side effects associated with these drugs, and know how to mitigate them, to ensure that their patients get the greatest benefit.
A large number of tyrosine kinase inhibitors (TKIs) targeting specific receptors have been approved over the last five years for many different types of cancer.
These agents inhibit kinase enzymes that act as ‘on’ or ‘off’ switches in many cellular activities, including proliferation, apoptosis, metabolism and transcription.
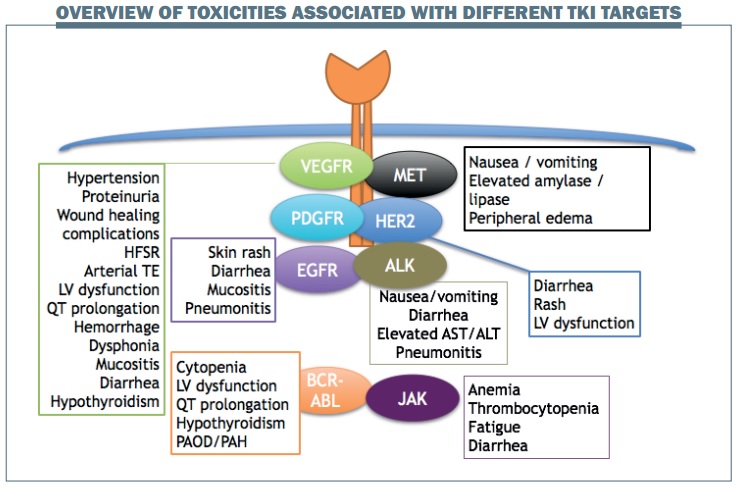
Different classes of TKIs targeting specific receptors are typically associated with particular toxicities (see figure below):
- EGFR inhibition is generally associated with skin rash, diarrhoea, mucositis and, less frequently, pneumonitis.
- VEGFR inhibition leads to hypertension, proteinuria, wound healing complications, hand-foot skin reaction (HFSR) and also some vascular complications such as arterial thromboembolism and left ventricular dysfunction.
- HER2 inhibition is associated with diarrhoea and rash, which are the most common toxicities associated with TKIs, and may also cause left ventricular dysfunction.
- ALK inhibition is most commonly associated with gastrointestinal toxicities such as nausea, vomiting and diarrhoea, some laboratory abnormalities such as elevated aspartateaminotransferase (AST) and alaninetransaminase (ALT) and, less commonly, pneumonitis.
- BCR-ABL kinase inhibition typically causes cytopenia, in addition to cardiac abnormalities and hypothyroidism.
EGFR inhibitor-associated toxicities
Rash, diarrhoea and mucositis are very common toxicities with EGFR TKIs. Rash is one of the most common toxicities in patients treated with EGFR inhibitors. Results for the adverse event profile in the LUX-LUNG 3 study that resulted in the approval of afatinib in non-small-cell lung cancer (NSCLC) showed that 89% of patients treated with the agent developed rash, with 16% having rash that was grade 3 or higher (JCO 2013, 31:3327–33). Other common side effects of afatinib were: diarrhoea (95% of patients), stomatitis (72%), paronychia (57%) and dry skin (29%).
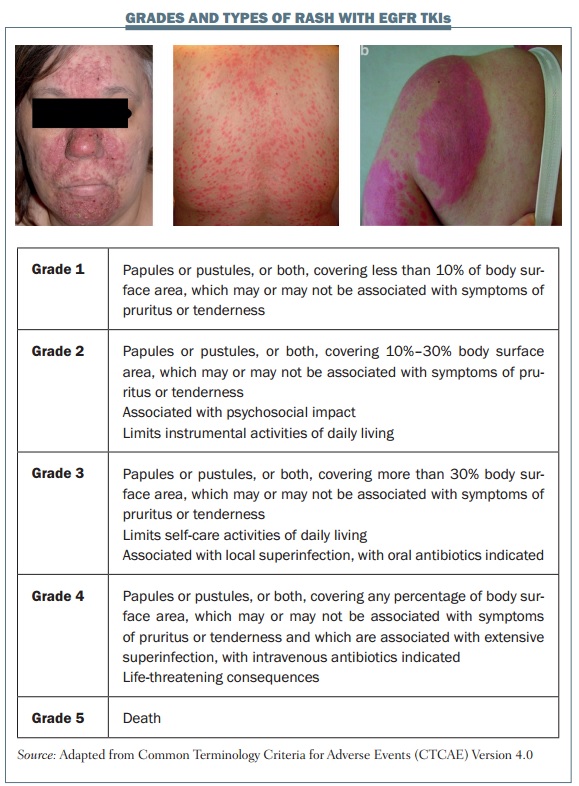
Rash with EGFR TKIs
Skin adverse effects with EGFR inhibitors are very common and we must be aware of them to improve the care of our patients. The figure below shows the grades and different types of rash associated with EGFR inhibitors. Several types of rash can occur, including acneiform or pustular rash (left) but rash can also have a more generalised distribution and bemaculopapular (centre). Rash can also be triggered by sun exposure, resulting in a photosensitivity rash (right). Acneiform rash is the type of rash most commonly associated with EGFR inhibition. It is defined as the eruption of papules or pustules and typically occurs on the scalp, upper chest and back. It improves over time if medication is continued, and resolves fully when treatment is discontinued.
All treatment-related toxicities in clinical trials are rated according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, and this is also useful in clinical practice. These criteria use the body surface area affected by rash as the main parameter to discriminate between the different grades: up to 10% for grade 1; 10–30% for grade 2, which may also have associated symptoms; and more than 30% for grade 3 rash, which limits self-care activities and may also be associated with local infection.
However, there are some limitations to using these criteria to classify EGFR TKI rash. Typically, in terms of assessing body surface area affected, EGFR-associated rash occurs on the face, trunk, scalp and the upper torso, and the NCI criteria do not take into account the severity of rash complications, such as oozing, burning, crusting or disfigurement. These are important factors to consider in order to give appropriate medication and in making decisions on when to stop EGFR inhibitor treatment or lower the dose.
Before initiating EGFR inhibitor therapy, several preventive measures can reduce the risk of skin rash. Areas of dry skin should be moisturised twice daily, because good hydration can prevent TKI-associated rash. Patients should minimise sun exposure and use a sunscreen with a protection factor of at least 15 to prevent photosensitivity rash. Patients should also avoid products that dry out or irritate the skin, such as soaps or alcohol-based perfume products.
The figure below shows a composite of several treatment frameworks for managing TKI-associated rash. The dose of EGFR TKI should be changed only in patients with grade 3 rash. For patients with grade 1 or 2 rash, I usually continue EGFR treatment and use topical treatment for their rash. Patients with mild skin rash may need no intervention, but topical hydrocortisone or clindamycin are reasonable options even if the rash is only grade 1.
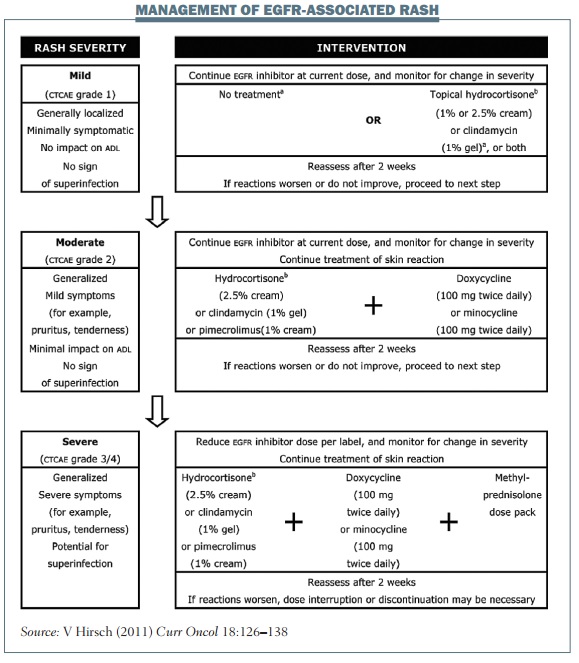 Topical hydrocortisone or clindamycin should be used to treat grade 2 rash, and oral antibiotics such as doxycycline or minocycline should be considered if patients have pustules or are beginning to develop an infection. Similar topical and oral treatment should be used in patients with grade 3 rash, with the addition of a short course of oral corticosteroids (typically 10–14 days) if the rash is really troublesome. Patients with EGFR-induced rash should be reassessed after two or three weeks of treatment and offered the next step in treatment if the previous therapy has failed to control the rash.
Topical hydrocortisone or clindamycin should be used to treat grade 2 rash, and oral antibiotics such as doxycycline or minocycline should be considered if patients have pustules or are beginning to develop an infection. Similar topical and oral treatment should be used in patients with grade 3 rash, with the addition of a short course of oral corticosteroids (typically 10–14 days) if the rash is really troublesome. Patients with EGFR-induced rash should be reassessed after two or three weeks of treatment and offered the next step in treatment if the previous therapy has failed to control the rash.
EGFR TKIs have long half-lives, so the management of adverse skin reactions should continue until they have resolved, even if treatment is discontinued or the dose reduced. Once a skin reaction has sufficiently resolved, typically to grade 1 or no rash, treatment should be restarted or the dose increased to the initial dosage, and you can expect this toxicity to remain well managed.
Diarrhoea with EGFR inhibitors
Diarrhoea is another toxicity commonly associated with EGFR inhibition. The toxicity profile for the ZETA study with vandetanib showed that diarrhoea was the most common adverse event, affecting 56% of patients and reaching grade 3 or higher in 11% (JCO 2011, 30:134–141). Importantly, diarrhoea in patients treated with vandetanib can be associated with colitis.
Vandetanib may also be associated with QT prolongation, so it is very important to check blood electrolytes and ECGs in patients with very profuse diarrhoea, to check there are no imbalances that may potentiate other adverse events of vandetanib.
The first step in management is to investigate other potential causes of a patient’s diarrhoea, including: other medications they may be taking, such as laxatives, stool softeners or antibiotics; lifestyle factors, such as excessive dietary fibre or lactose; and infectious causes of diarrhoea.
Diarrhoea associated with EGFR TKIs is usually mild to moderate, and early management is essential to prevent dose reduction or discontinuation of treatment. Management is similar as for diarrhoea associated with chemotherapies. Antimotility agents such as loperamide should be initiated on appearance of mild diarrhoea, and patients should avoid foods that cause symptoms, and eat a simple ‘BRAT’ diet (bananas, rice, apple sauce and toast). Patients should be advised to drink approximately three litres of liquids a day to minimise the risk of dehydration.
Patients should return to the clinic for further assessment if their diarrhoea persists despite up to 20 mg loperamide per day. For patients with persisting grade 2 or 3 diarrhoea, interruption of treatment may be considered to allow symptoms to improve. The dose of treatment may also be reduced to control diarrhoea. If diarrhoea fails to resolve after dose reductions or discontinuation, octreo-tide may be considered in some cases, but it is very rarely needed to manage EGFR TKI diarrhoea, and there is little evidence that supports its use in this situation.
Mucositis with EGFR inhibitors
Mucositis is another common side effect with EGFR TKIs. In the LUX-LUNG 3 study with afatinib, 72.1% of patients had mucositis, which was grade 3 or higher in 8.7% of the patients (JCO 2013, 31: 3327–34). The figure below shows some examples of mucositis and explains the management of different grades.
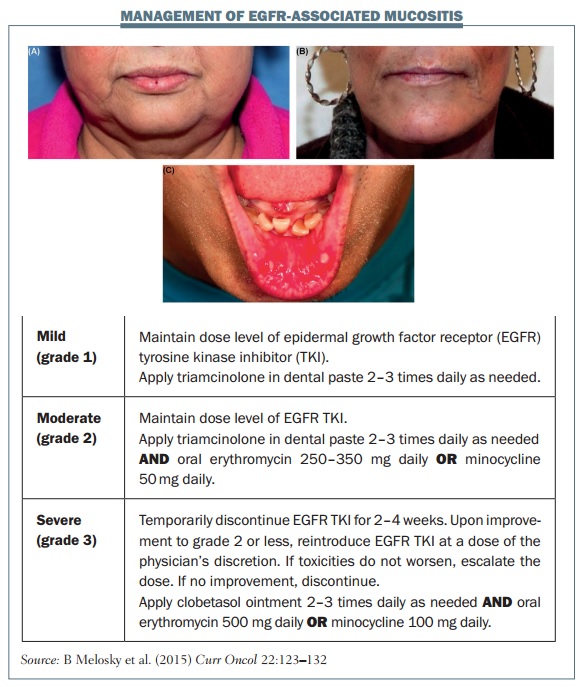 Several general measures are very useful for managing mucositis. Before the start of treatment, patients should have an oral hygiene check-up. Advise the patient to brush their teeth and tongue with a soft-bristled brush in addition to flossing and rinsing with normal saline.
Several general measures are very useful for managing mucositis. Before the start of treatment, patients should have an oral hygiene check-up. Advise the patient to brush their teeth and tongue with a soft-bristled brush in addition to flossing and rinsing with normal saline.
Oral mucositis generally starts as a tingling sensation in the mouth, with patients becoming very sensitive to food and beverages, and eventually developing ulcers. Grade 1 mucositis is a minor symptomatic inflammation of the mouth; grade 2 causes some pain but eating and drinking are tolerable; patients with grade 3 mucositis can experience severe pain that prevents them eating or drinking.
If a patient develops mucositis in the mouth, they should rinse their mouth out every two to three hours. Mouthwash or bicarbonate can be useful for grade 1 mucositis. Patients with grade 2 mucositis should apply triamcinolone paste two to three times daily, and mouthwashes that include corticosteroid are very useful for treating mouth ulcers that may develop. If a patient develops grade 3 mucositis you should typically stop EGFR inhibitor treatment for two to four weeks. Oral anti-biotics or mouthwashes that combine corticosteroids with antibiotics are very useful. Don’t forget to give analgesics, because grade 3 mucositis can be very painful.
Toxicities associated with VEGFR inhibitors
Hand-foot skin reaction (HFSR), hypertension, left ventricular dysfunction and elevated liver enzymes are common toxicities associated with VEGFR inhibitors.
Hand-foot skin reaction
The incidence of HFSR in the CORRECT trial with regorafenib in colorectal cancer was 47%, with grade 3 HFSR in 17% of patients (Lancet 2013, 381:303–312). With another VEGFR inhibitor, cabozantinib, the incidence of HFSR was 50% in the MTC study (JCO 2013, 31:3639–46). HFSR associated with VEGFR inhibitors and other targeted treatments show some particular features that are different to HFSR associated with traditional cytotoxic chemotherapies.
In HFSR associated with multikinase inhibitors, patients typically complain of dysaesthesia with tingling that develops into burning over a few days. They develop bilateral, painful erythema and also large, intense blisters that evolve eventually into hyperkeratosis. The pain may be completely out of proportion to the clinical appearance of the lesions. Symptoms typically occur at pressure points, such as the palms of the hands or the soles of the feet, particularly the heels and the metatarsal hand areas, and also the elbows.
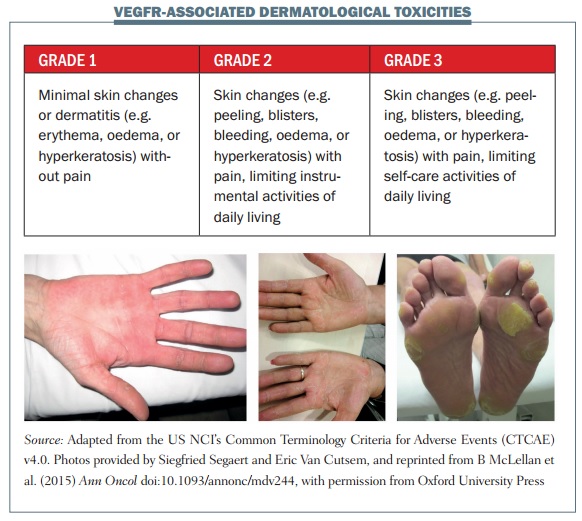 The figure above shows some examples of dermatological toxicities, with minimal skin changes and mild erythema in the first image and severe hyperkeratosis and erythematous areas associated with pain in grade 3 HFSR in the third image.
The figure above shows some examples of dermatological toxicities, with minimal skin changes and mild erythema in the first image and severe hyperkeratosis and erythematous areas associated with pain in grade 3 HFSR in the third image.
HFSR usually occurs early, typically within four weeks of starting regorafenib treatment, and most commonly within the first two weeks. It is not life-threatening, but it can negatively affect a patient’s quality of life, so it is important to manage HFSR carefully. Prompt initiation of management can reduce the severity and the duration of HFSR, and close monitoring during the first two cycles of treatment is crucial. I assess patients each week during the first four to six weeks of treatment, and treat promptly if mild symptoms of HFSR appear.
In terms of management, for grade 1 HFSR the skin should be kept well hydrated, but humidity is not helpful. If symptoms reach grade 2, TKI treatment may be stopped in some cases. Hyperkeratosis should be controlled, the skin kept moisturised and discomfort relieved with analgesic. The goals for treating grade 3 HFSR are to reduce symptoms and the impact on the patient’s quality of life. Each TKI has specific recommendations for discontinuing treatment and reducing the dose, so it is important to follow these in managing symptoms of HFSR.
Hypertension with VEGFR inhibitors
Hypertension is the most common cardiovascular-related toxicity associated with several VEGFR inhibitors such as axitinib and lenvatinib. In the SELECT trial with lenvatinib it was the most common side effect, affecting 67.8% of patients (NEJM 2015, 372:621–30). Typically it occurs early, within three to four weeks of starting treatment.
Before starting TKI therapy, a patient’s blood pressure should be controlled for approximately one week. Their blood pressure should be carefully monitored, with weekly measurements in the first cycle of treatment, and then every two to three weeks, or more frequently if required. It is not recommended that treatment is stopped for any grade of hypertension, but in a patient with grade 2 or 3 hypertension that is difficult to control, it can be useful to stop the TKI and get the blood pressure back under control.
Patients who develop stage 1 hypertension or who have >20 mmHg increase in diastolic blood pressure should start hypertensive therapy and you should consider modifying the dose or add a second antihypertensive to achieve recommended blood pressure, following hypertension guidelines.
Left ventricular dysfunction (LVD) with VEGFR inhibitors
Left ventricular dysfunction (LVD) is more common with sunitinib and sorafenib, although it may occur with axitinib. You should consider measuring left ventricular ejection fraction (LVEF) at baseline, but the ideal time for cardiac follow-up is not yet established for these drugs. Ask patients about cardiovascular risk factors that would mandate LVEF assessment before starting treatment.
When symptoms occur, or when LVEF decreases to less than 50% or by more than 10% from baseline, interrupt the treatment or reduce the dose. Good communication and collaboration with a cardiologist is very important in managing these patients. The effect on LVEF is generally reversible on stopping treatment.
Elevated liver enzymes
Elevated aspartateaminotransferase (AST) or alaninetransaminase (ALT) is a common side effect with many TKIs. The first step in management is to rule out other causes, such as other drugs or infections. Monitor liver enzymes as clinically indicated throughout treatment, and measure ALT and AST levels before each cycle. Drug interruption or dose adjustments are useful and can generally manage this side effect well.
Pneumonitis associated with ALK inhibitors
Non-infectious pneumonitis is associated with ALK inhibitors. It is not very common but there may be potential issues, making it important to consider. In the phase I trial of ceritinib that led to accelerated approval, pneumonia and pneumonitis were the most frequent adverse drug reaction s that led to discontinuation, affecting 1% or more of patients (NEJM 2014, 370:1189–97).
It is difficult to distinguish whether pneumonitis is associated with treatment or due to disease in lung cancer patients, but it is important to be aware of the potential association with ALK inhibitors to guide optimal management.
Drug-induced non-infectious pneumonitis is a diagnosis of exclusion. Patients may have other conditions that may cause dyspnoea and cough. General guidelines on management recommend:
- Rule out other causes for respiratory distress: infections, occupational, recreational or environmental exposures; specific respiratory disorders such as asthma; and systemic diseases.
- Work up: Chest CT scan, bronchofibroscopy to rule out infectious causes, DLCO measurement (if baseline values are available).
- Treatment: stop ALK inhibitor treatment. Treat with cortico-steroids and supportive treatment, such as bronchodilators, supplementary oxygen, and mechanical ventilation.
- For cediranib: the recommendation is to permanently discontinue treatment in the event of any grade of pneumonitis.
Haematological toxicities with ABL and JAK inhibitors
Thrombocytopenia, anaemia and neutropenia are typically associated with ABL and JAK inhibitors. Blood count alterations are common with these agents, with 42% of patients having thrombocytopenia and 28% anaemia with bosutinib in a phase I/II study (Blood 2014, 123:1309–18).
These toxicities are managed with dose reduction or temporary discontinuation. Specific guidelines in the summary of medicinal product characteristics for ponatinib and bosutinib set out when to reduce the dosage, when to restart and when to discontinue. Some patients need blood transfusions or growth factors, but dose reductions can usually manage these toxicities.
Take-home messages: preventing and managing TKI-associated toxicities
Toxicities with new (and old) TKIs may be predictable, with some class effects such as rash with EGFR inhibitors and hypertension with VEGFR inhibitors.
Awareness of these toxicities can decrease their seriousness by enabling prevention and prompt management, which can improve patient care and quality of life.
Maintain good communication with patients, listening to what they tell you and asking about potential side effects.
The mainstay of management for TKI toxicities is intensive supportive care, dose holding and, if needed, dose reduction.






Leave a Reply