
Whether you work at a cancer unit, a cancer centre or a comprehensive cancer centre, applying for OECI accreditation is a good way to find out how your institute compares with the best, and get advice on how to address any shortcomings.
The European School of Oncology presents weekly e-grandrounds which offer participants the chance to discuss a range of cutting-edge issues with leading European experts. One of these is selected for publication in each issue of Cancer World.
In this issue, Mahasti Saghatchian, of the Institut Gustave Roussy, in Villejuif, France, reviews the OECI programme for accrediting cancer centres, which she helped develop and now chairs. Gordon McVie, from the European Institute of Oncology in Milan, Italy, poses questions arising during the live presentation. It is summarised by Susan Mayor.
The recorded version of this and other e-grandrounds, is available at www.e-eso.net
The fragmentation of academic research is a major organisational problem in Europe. There is also a clear need to improve translational research and to overcome differences experienced by patients across Europe in access to diagnostics and treatment, which feed into disparities in therapeutic outcomes.
To address these issues, the Organisation of European Cancer Institutes (OECI) – a body dedicated to improving research collaboration among Europe’s cancer centres – identified the need to develop a system for monitoring the research and care offered to patients, and also to find ways to harmonise patient care and share the knowledge that we are developing to improve the standard of care. To this end, the OECI developed a tool for assessing and benchmarking the care and research being carried out in cancer centres.
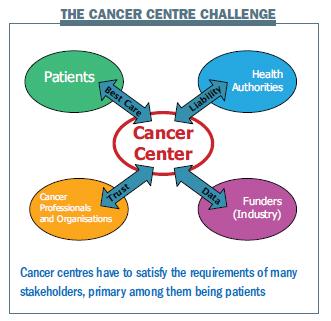
The challenge for cancer centres is to meet all the requirements of the different stakeholder. The key stakeholder is the patient, and a central aim for any cancer centre is to provide patients with the best care. The challenge for the OECI was, first and foremost, to develop a tool that would allow a cancer centre to ensure it was achieving this aim. Cancer centres are also answerable to health authorities, and we wanted to develop a tool that would help centres to collect an evidence base to prove they are providing good-quality care.
We also wanted the tool to help build the trust of cancer professionals and other organisations in the care and research being performed by cancer centres. Building the evidence base on the performance of each cancer centre could strengthen this trust. Finally, funders, including industry and the health authorities, want data on activity, outcomes and production in terms of activity. We wanted our accreditation system to be able to help cancer centres provide this.
 A supportive approach
A supportive approach
Most quality assessment programmes are regulatory measures imposed by an external authority, and are usually compulsory. We developed the OECI quality assessment programme as a supportive measure for cancer centres, and it is voluntary. It has been designed and developed internally by people from cancer centres. Peer review is performed by people from cancer centres and visits are chaired by the director of another cancer centre. The standards that we have set have been developed and are revised every four years by people from the centres.
Gordon McVie [GM]: You work at the Gustave Roussy, one of the most prestigious cancer centres in Europe. Why does your centre want to get OECI accredition?
Mahasti Saghatchian [MS]: We like to think we are one of the best, but when we entered the programme we still felt anxious about people visiting us. We prepared for the accreditation and visit very seriously and found it a very rewarding experience. Having a group of auditors visit us and assess what we do from an external point of view was reassuring. It is not enough to claim that you are a comprehensive cancer centre, you need to prove it to your peers and collect the necessary evidence.
Gordon McVie [GM]: It’s a little bit like submitting research to a journal. As an author you think your research paper is great, but we need other people to look independently and assess its value.
MS: Different countries have different assessment and accreditation systems in place. In France we have mandatory accreditation for hospitals in general, but not specifically for cancer centres. Here at the Institut Gustave Roussy, we are going to be the only cancer centre accredited by the OECI.
GM: The process is similar to that which the US cancer centres have gone through, and provides a great deal of credibility.
The OECI tools
The main tools that the OECI has developed are the accreditation and designation programme and the EurocanPlatform Network of excellence, both of which are supported by the European Commission and focus more on centres dedicated to translational research.
In the accreditation and designation programme, we have developed a tool similar to other accreditation programmes in its design, with a set of quantitative and qualitative standards integrated in a database, a report and a peer review system. The programme operates at three levels: the comprehensive cancer centre, the cancer centre, and the cancer unit. A pilot among OECI centres showed these are the three most frequent types of centres. We developed specific criteria for each of these. The programme includes standards and a database with a set of qualitative and quantitative data. We write a report and an improvement plan for each centre, which are reviewed by the accreditation board and then discussed with the centre.
Developing the programme
The accreditation programme has been running now for 10 years. The idea was first discussed in 2002. Then in 2005 the project was launched as an accreditation programme setting standards for cancer centres. Subsequently we realised that this was not sufficient and we would need different assessments for different types of centres, differentiating between larger or smaller centres for care or research and comprehensive cancer centres. We then developed the criteria that allowed us to have a designation system for the cancer centres, and launched the merged accreditation and designation programme in 2008.
We are at the end of the first version of the accreditation and designation programme and will be launching the second version of the programme in 2013. The standards are currently being revised and we are reviewing the quantitative data that we ask centres to provide. We are also working on a specific programme for prostate cancer centres in collaboration with the European School of Oncology, which has developed guidelines for prostate units. The OECI is now turning these guidelines into an accreditation system for prostate cancer units.
The OECI accreditation and designation programme is run by a board of people from cancer centres and has a management unit of people paid by the OECI to coordinate and run the programme. There is also an accreditation committee, with 10 people from various cancer centres, which is chaired by Chris Harrison from the Christie Cancer Centre in Manchester, UK. This committee reviews all the reports provided by the auditors and makes recommendations to the board.
The programme uses a set of quantitative and qualitative questionnaires, which are currently being revised. These are integrated into an electronic tool available with a username and password and there is also a manual in paper format. When a centre participates in the programme, all answers to the questionnaires are entered into the database. A group of auditors, all from OECI cancer centres, carry out peer review
visits.
 Participating centres
Participating centres
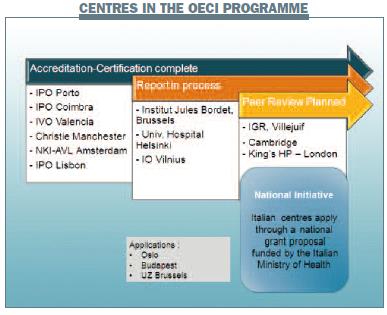
Centres from all over Europe are taking part in the OECI programme. The first centres to enter the programme were in Portugal. Their health ministry asked all cancer centres to participate in an international accreditation programme and, after discussing whether to follow the US or European accreditation programmes, three centres opted for the OECI programme. Comprehensive cancer centres, smaller cancer units and university centres have all taken part in the OECI programme, and experience has shown it is applicable in all of these types of centres.
Of note, the Italian Ministry of Health has decided to fund all comprehensive cancer centres in Italy to go through the OECI accreditation programme. The nine Italian centres will go through the programme over two years. We think this is a good example of a country deciding to go for a European accreditation programme, and Italy is pioneering this.
GM: How are different types of cancer centres defined? I know about comprehensive cancer centres because I am based at one in Milan. The European Institute of Oncology has a hospital and a large laboratory and patients are seen by multidisciplinary teams, so we are a comprehensive cancer centre. The Christie cancer centre, in Manchester, and the NKI-AVL, in Amsterdam, have similar facilities. Is the IPO [Instituto Portugués de Oncologia] in Lisbon a comprehensive cancer centre or a cancer centre?
MS: It is a cancer centre, but not a comprehensive centre, because it does not have enough research activities.
GM: Then what is a cancer unit? Have you looked at the cancer unit at the University Hospital of Helsinki?
MS: None of the centres currently in the programme is a cancer unit. We are currently in a one-year pending period for Helsinki. They applied as a comprehensive cancer centre, but it appears that they are a cancer department in a large hospital. Although they have a large amount of research and their research is of high quality, we considered that their research and care was not sufficiently integrated, and that their management and strategy in oncology were not clearly separated from the rest of the work of the university hospital. We considered that Helsinki could not be designated as a comprehensive cancer centre because they did not have the organisation, strategy or logistics of a comprehensive cancer centre. We offered them a one-year pending period to reorganise and develop the processes needed to achieve these.
GM: This strikes me as a very important example of the last part of the whole accreditation programme – the improvement plan. Cancer centres really appreciate that these accreditation visits are not just about checking that everything is OK or being critical, but are about saying, ‘This is how you are now, what are you going to do to make things even better?’
Focus of the programme
The accreditation programme focuses on what is critical to cancer care. This includes:
- planning and organisation of integrated care
- multidisciplinary teams and care
- integration and translation of clinical and basic research into care
- education for professionals
- the experience and involvement of patients
- monitoring and organisation of quality improvement.
The accreditation process
Application and designation screening
When a centre applies to the programme, the first step is to define whether you are applying as a cancer unit, a centre or a comprehensive cancer centre. Comprehensive centres have to provide data related to research, while cancer units and centres do not. Differences are otherwise based on volume of infrastructure, budget and activities. All centres then complete a self-evaluation period providing the required information, and the OECI makes a ‘go or no-go’ decision, advising the board on whether a centre is ready for peer review based on the information provided. This process avoids organising a peer review in a centre that is not ready.
Explanatory visit
An explanatory visit takes place immediately after the application and ensures that everyone in the centre is aware of the process, what will happen, and the information required. The OECI helps centres develop an action plan. We have found that centres have to dedicate one full-time person for a six-month period to collect the required documents and data.
Self-assessment period
In the self-assessment period, centres are required to collect documents, annual reports and data on quality, education, comprehensiveness and patients. It usually takes at least six months. We have found that gathering data on research activities is particularly difficult.
Electronic self-assessment tool
The e-tool includes a quantitative and the qualitative questionnaire. Qualitative questions are scored to assess the centre’s degree of compliance to the standards, which are as detailed as possible in each question. Centres are asked to send reports or documents to support their answers. These can be uploaded onto the system. The tool automatically shows the centres any criteria where they are not fully compliant with the required standards, and helps to build improvement plans. Quantitative questions ask for numbers relating to infrastructure, resources etc. Once all the figures have been submitted, the auditors review all the data, and then prepare for their visit.
Peer review visit
Trained auditors spend two full days on site, during which time the centre’s activities should continue as normal. They can visit anywhere in the centre and carry out interviews with staff or even patients. Based on the self-evaluation reports and what they have seen, the audit group gives a first summary report about the accreditation, the designation, and points that can be improved. The chairman of the audit group is always a director of an OECI centre; two auditors are specialised in a relevant field of oncology, one is a quality manager and other personnel are from the OECI.
Peer review report
The peer review report is developed following some exchanges with the centre. The final report highlights the centre’s strengths and the opportunities for improvement. When the report has been agreed on, a decision is made on accreditation and designation. If accreditation is given, it is valid for four years.
The cost for the full process of assessment and designation is €30,000 for a comprehensive cancer centre, which is paid to the OECI. The cost is lower for cancer centres and cancer units, at €25,000 and €20,000, respectively.
EurocanPlatform research programme
The EurocanPlatform programme is a research programme aimed at developing a designation system for excellent comprehensive cancer centres and comprehensive research centres, and outcome indicators for translational research. Proof of excellence includes recent research reports, grants awarded, and ongoing or completed research programmes and their outputs. The programme is also developing excellence indicators that will help us measure impact on patients.
Summing up
We hope that the OECI accreditation programme and EurocanPlatform will help us to reduce fragmentation and increase harmonisation across cancer centres. The aim is for centres to work more closely together, with people visiting each other’s centres and learning from each other in a network that supports competition and collaboration.
GM: How do you accredit a paediatric oncology centre? Would a paediatric oncology centre have to be accredited as part of an adult oncology network or are you anticipating that there could be stand-alone paediatric oncology centres accredited in the future by OECI?
MS: Paediatric oncology is facing the same issues as adult oncology. We are collaborating closely on this with a network of European paediatric oncology personnel. I think we could apply the accreditation model to paediatric units, but we might have to adapt some questions or add further standards related to paediatric care.
GM: What does a comprehensive cancer centre do for a patient in a district general hospital perhaps 100 kilometres from the centre?
MS: Comprehensive cancer centres are very important for other hospitals. Not all patients can be managed within the walls of the cancer centre. One of the very important standards is that centres must have networks, where they work with other professionals taking care of patients. It is the duty of the cancer centre to organise this network and the education of professionals within it.





Leave a Reply