Winning the argument for expanding and upgrading radiotherapy facilities is not easy in the present economic climate.
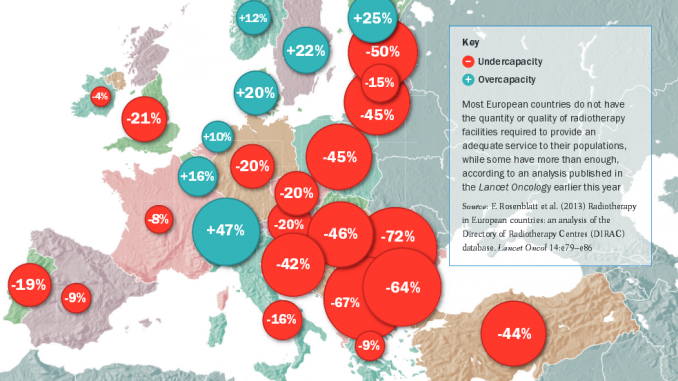
How many radiotherapy mach-ines are there in each country in Europe? It might seem an easy question to answer given that it is hard to overlook a large radiotherapy suite complete with several linear accelerators (linacs), and ancillary equipment such as CT, MRI and PET scanners, and treatment planning workstations. Earlier this year, Lancet Oncology (vol.14, pp 79–86) carried a lengthy paper that looks to have these numbers well documented in terms of actual installations and estimated need, from a group reporting on the European portion of the Directory of Radiotherapy Centres (DIRAC) database, which is managed by the International Atomic Energy Authority (IAEA). The authors conclude that there is “a substantial disparity in the availability and organisation of radiotherapy services between countries”.
The inventory attracted a lot of attention around Europe. It was commented on by a number of professional societies and cancer organisations, and also featured in Nature Reviews Clinical Oncology, because it has been some years since a similar survey was published, and the headline finding is a call for modernisation of facilities, particularly in East and South-East Europe.
But there was also some criticism about the accuracy of figures from various countries. Further, the paper, and a detailed reply from colleagues at Europe’s radiotherapy and oncology society, ESTRO, raise important questions about whether these data alone are good enough to inform policymakers, or whether new value and cost-effectiveness indicators are needed to make investment decisions in what tend to be very expensive facilities, both in terms of equipment and personnel.
Previous data came from the QUARTS (quantification of radiotherapy infrastructure and staffing needs) project, published in 2005, and carried out by ESTRO. Both projects have uncovered unmet needs in radiotherapy, mainly on the basis of counting centres and machines and then estimating from cancer incidence and population in each country whether there is sufficient capacity to deliver required treatments, given that a proportion of patients should have radiotherapy as part of their treatment (the DIRAC paper says “roughly 45–55% at some point”).
The analysis of the DIRAC database was carried out by the European Network for Information on Cancer (EUNICE) over several years. EUNICE covers 33 countries, including all members of the European Union plus others such as Iceland and Turkey. DIRAC itself has a long history as a global listing of radiotherapy facilities, dating back to 1959, and now lists 137 countries and more than 7600 radiotherapy centres. In Europe the authors found 1286 active radiotherapy centres as of July 2012.
The authors calculated indicators by counting the number of ‘teletherapy’ machines per centre (with linacs being by far the most common type of equipment), as well as brachytherapy units. The picture that emerges is one of varying levels of concentration of services, with some countries such as the UK and the Netherlands having facilities centralised in fewer, large units. They also looked at the adequacy of radiotherapy capacity in each country. Using benchmarks from the earlier QUARTS project, and total population figures for each country, they calculated the number of machines that would be required to provide ‘average’ and ‘minimum’ levels of service, and compared that with the actual number of machines, to show the level of unmet need. Figures range from 72% in Romania (i.e. Romania has around one-quarter [28%] of the radiotherapy machines needed to serve its population), to –47% in Switzerland (meaning Switzerland has almost 50% more machines than it requires).
There are a number of limitations with this study, as the authors acknowledge. The benchmarks they used are crude and the QUARTS benchmarks are old, and don’t take into account possible new national guidelines. The report also does not take into account the epidemiological cancer profiles of each country. The authors note, in particular, that demand for radiotherapy depends heavily on breast and prostate cancer incidence, which could be affected by screening programmes. The study also doesn’t address quality issues – it is mainly a counting exercise – and it was not able to include data on personnel, because of the difficulties in defining just who is a radiation oncologist and other roles such as physicists and technicians in a manner that was applicable across countries.
Cost-effectiveness models
In a detailed comment on the Lancet Oncology article, colleagues from ESTRO query the accuracy of the data in DIRAC, finding some discrepancies when checked against figures from national radiation oncology societies. But the more substantial issues they raise is that more reliable data now exist on how radiotherapy is used, and that many of the acknowledged shortcomings of the DIRAC analysis – such as epidemiology, staffing and economics – are being addressed in ESTRO’s Health Economics in Radiation Oncology (HERO) project.
Among the team running HERO is Yolande Lievens, head of radiation oncology at Ghent University Hospital, Belgium, who has a background in health economics, and did her PhD on costing and value for money in radiotherapy. That study focused on her own (previous) hospital, comparing it with another in the Netherlands to see whether the methodology she had developed was translatable to other centres.
Lievens says that a first step in Belgium, as in other countries, has been defining what the real costs of providing radiotherapy are, so that the appropriate reimbursement can be made and planned for. “But now, as with the drugs side, authorities are also asking what the cost-effectiveness is – with radiotherapy evolving very quickly we have to provide information on the value for money of novel treatments compared with standard ones,” she says.
A paper by Lievens and colleagues at University Hospitals Gasthuisberg in Leuven, Belgium, entitled ‘The cost of radiotherapy in a decade of technology evolution’ (Radiother Oncol 2012, 102:148–153), shows how a costing model can be implemented in a centre as technology advances. In the decade under discussion, costs roughly doubled, with contributing factors being complex treatments and new techniques, such as intensity-modulated and image-guided radiotherapy (IMRT/IGRT).
As Lievens explains, while the aim of the QUARTS study had been to provide a blueprint of equipment and personnel at a national level across Europe, and estimate the need and unmet need for radiotherapy, it had also aimed to carry out an economic analysis along similar lines to the one in her study. However, the project funding ran out. Now, in line with ESTRO’s mission to provide more support for national societies, the HERO project has taken on that task. “And there are many countries that still face difficulties in arriving at a correct reimbursement for radiotherapy, hence the importance of providing evidence on the cost-effectiveness of our treatments,” she says.
HERO is now revisiting the baseline data on radiotherapy units, this time in more countries, but there is also a formal arrangement with the Collaboration for Cancer Outcomes Research (CCORE), an Australian project that has come up with a more robust gauge of how many patients should be given radiotherapy (the basic figure is 52%, which is at the higher end of the DIRAC estimate). “This will allow us to evaluate needs for radiotherapy based on the incidence of cancer in European countries,” says Lievens. “What we want to show first is how much it costs to deliver radiotherapy based on resources as they are now, and in countries where there is under-resourcing what would be the cost of installing new technology such as IMRT to an optimal level. Moreover, we want to present a methodology for cost-effectiveness of radiotherapy at the national level to support the implementation of novel technologies and improve treatments in certain cancers.”
“We want to show what would be the cost of installing new technology such as IMRT to an optimal level”
The HERO project
There are four main steps in HERO:
- Mapping resources
- Estimating optimal resources to meet needs
- Cost accounting at national level – so far this has mainly been done only at departmental level, as with the paper in Leuven, and
- Building cost-effectiveness or economic evaluation models, again at national level.
“We want to compare countries, and our aim is to develop the costing and cost-effectiveness models in core countries before rolling it out across the whole of Europe,” says Lievens. The project also aims to benchmark radiotherapy against other oncology treatments from an economic standpoint.
Some countries, such as the UK, have detailed programmes on radiotherapy needs and costing, derived from data specific to their healthcare systems, says Lievens, but these are not readily translatable to a European model. ‘Radiotherapy services in England 2012’, a good report that shows how Britain’s NHS is approaching radiotherapy, notes that substantial new capacity is still needed. In addition to setting out national targets, there is a model called Malthus for simulating radiotherapy demand at local level, which also uses CCORE. “But most countries are not as far advanced,” says Lievens, “and we want a methodology that is applicable to all, and especially to those that cannot at present go into such detail on their own.”
HERO, she adds, is nearing the end of the information gathering stage, surveying not only the number and type of equipment and personnel, but also cancer incidence and the proportion of patients treated with radiotherapy, and details of existing national planning guidelines and reimbursement systems, in each country.
The data collection is a challenge – even in a small country such as Belgium, national data on equipment and personnel were not available. “If we did not have these data in Belgium, you can imagine that in countries such as Germany and Italy, where there are many small private centres, it is even harder to collect the details. In each country our first task has been to find contacts who can and are willing to collect the data – and that is not easy. Take Belgium: at the time of the first data collection there were three radiotherapy societies. Two societies have merged since and all have changed presidents – so who is the right contact?”
ESTRO is employing a data analyst to check information, which should be finished in 2013. The needs analysis and cost calculations are also underway, while the work on cost-effectiveness will not start until 2014. The models, she says, aim to cover the changing complexity of treatments and do ‘what if?’ analysis of, for example, the cost and cost-effectiveness of introducing IMRT across a country, or the impact of starting a screening programme for breast cancer on the uptake of radiotherapy. “We hope that once the national radiotherapy societies see the advantages of HERO, we will be able to collaborate and collect data on a continuous basis,” she adds.
Countries need support to make the case not only for more facilities, but also training programmes. “And although lobbying is not part of the project we hope that by making the case for radiotherapy we will help bring it to wider attention in the public mind, as it is often not presented in a positive way – just when things go wrong or it is deemed unaffordable.”
Julian Malicki, director of the Greater Poland Cancer Centre in Poznan, has been involved with both QUARTS and HERO, and says benchmarks are particularly needed in countries such as his, where there is a shortfall of radiotherapy (the DIRAC study estimates 45% of the needs in Poland are unmet). “We did have a national cancer plan in 2005, under which the government allocated more resources to radiotherapy, but it is a 10-year programme that is nearing its end, and some say enough has been given to radiotherapy and cancer, and that other disciplines need more money now. Yes, the gap is narrower now than it was, which is why we need better data that projects like HERO will provide to convince the government to continue with investments in cancer.”
The province of Greater Poland has 3.5 million people served by two cancer institutes – one public, which is Malicki’s centre with eight machines, and also a private facility. Malicki says his centre is building two satellite units in cities up to 100 km from Poznan. “It has been very important to use data to justify these needs to our regional government,” he says. Cancer incidence does differ among countries, he adds, but not significantly, “and the best way to convince decision makers is to show them what a European optimal level of provision looks like. We need more investment not just to speed up infrastructure but also the number of specialists – in my medical university we have problems recruiting students who want to specialise in radiotherapy. HERO will also help to show what personnel we will need in the future.”
“The best way to convince decision makers is to show them what a European optimal level of provision looks like”
In Poland, he says, the national head of radiotherapy collects data on resources, but other data, such as on distribution of new cases of breast and prostate cancers, are less available at present. “We have made our submission to HERO, but the data do vary in accuracy. The cost-effectiveness model will be the most important for us, because we need to come to conclusions about how much we need for radiotherapy care.”
This practical assistance in gauging the adequacy of national radiotherapy capacity and building a strong cost-effectiveness case for investment where appropriate will doubtless be welcome throughout Europe’s radiotherapy community. Greater coordination between the IAEA, which produces the DIRAC directory of radiotherapy at a global level, and ESTRO’s HERO project could lead to less duplication of effort in Europe, and potentially open the way for other parts of the world to benefit from the HERO methodology and experience.





Leave a Reply