
Joining a clinical trial can be a lifeline for patients with few options open to them. But are outdated attitudes and practices preventing them from benefiting as much as they could?
The transformation in the nature of cancer trials over the past 20 years has been well documented. Gone are the days when phase I trials were about dying patients sacrificing themselves to test the toxicity of experimental therapies, with only the slightest hopes of deriving benefit themselves. Today, when so much more is known about targets and mechanisms before a drug enters human trials, early-phase trials are much more about learning who benefits, at what stage, at what dose and possibly even in what combinations.
What are the implications for optimal treatment of patients who have an incurable cancer? Should doctors and patients be rethinking the role that joining a phase I trial can play within their overall treatment strategy?
Jean-Charles Soria, who heads the dynamic Department of Drug Development at the Gustave Roussy cancer centre in Paris, believes that large numbers of patients with advanced cancer are missing opportunities to improve and extend their lives because their doctors fail to grasp the possibilities now offered by phase I trials. His message to medical oncologists is: “Don’t wait until your patient runs out of options before suggesting a phase I trial”. This discussion should be started early in the disease trajectory, he adds, ideally as soon as a patient becomes resistant to first-line therapy.
“I look at the attitude of most clinical oncologists towards phase I and, to be provocative, I would say they consider phase I to be an alternative to going to Lourdes or to Fatima – desperate, extreme cases. They don’t tend to consider their patients for phase I early on in the course of their disease, and this is a major problem.”
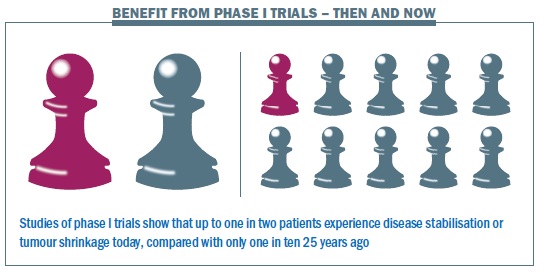
Twenty years ago, he concurs, this would have been a reasonable attitude, because only about 10% of patients derived any benefit, and only 1% showed an objective response (tumour shrinkage), while toxicity was seen as potentially significant. Since the mid 1990s, with the advent of molecularly targeted agents, and more recently with the advent of new immune checkpoints, he points out that the activity level in phase I trials is much higher – “very similar to any standard chemotherapy you would give in the third-line setting to any solid tumour.”
Soria cites a number of studies, including one from his own Gustave Roussy (Ann Oncol 2008 19:787–792) and another from the Royal Marsden (Br J Cancer 2008, 98:1029–33), that indicate that the objective response rate in phase I trials today is closer to 8–10%, with a further 40% showing stable disease. “Benefit for the patient can be in the range of one out of two, which is much higher than one out of ten.” Toxicity is also well managed. “The risk of death in a phase I trial – to take an extreme indicator – is 0.5 %. That is much lower than the risk of death from adjuvant chemotherapy in breast or lung, which is in the range of 1–3%.”
Even if this is a convincing argument in favour of joining a phase I trial, why not hold back until all standard lines of therapy have been exhausted? Soria draws an analogy with a game of chess, where you are trying to keep one step ahead of a cancer that can constantly evolve in response to treatment, and every move you make can limit what may be open further down the line. The benefit of not waiting too long before joining a phase I trial is that, whatever the outcome, the option of the standard second- or third-line treatment remains open, says Soria. If you choose the opposite strategy, a place on a phase I trial may not be available when you need it and other options have been exhausted.
He points out that demand for places on a phase I trial is now so high that there are not enough for all the patients who want to join.
“Once you have used up all the standard options, you have no alternatives except a phase I. If there is no slot – that’s it. The patient goes to palliative care or whatever. If you start thinking about phase I as soon as you are in the metastatic setting, when the first-line therapy has failed, then if by mischance when you ask for the slot there is none – you get multiple chances. You can ask again after the second, or third line.” Waiting too long can also damage your chances of being fit enough to join a phase I, adds Soria. “We know that if we give a drug to a patient whose kidney or liver are not functioning well then the risk of toxicity is huge.”
 It seems highly controversial to suggest that a patient could opt for experimental treatment in preference to an approved evidence-based therapy but Soria responds, “there is a difference between what is evidence based and what is scientifically or medically rational.” He gives the example of a patient with advanced melanoma that is not treatable with a BRAF inhibitor, and where ipilimumab has failed as first-line therapy. “What is my standard of care in second line? It is a lousy chemotherapy. What is my option in phase I? It is a PD1 inhibitor [a new class of immunotherapy currently showing great promise in melanoma]. Which do I choose? Anyone with a real mind would choose a PD1 because the likelihood of activity is 40%, while the likelihood of activity of standard chemotherapy is 10%. But that’s not evidence based, because the trial has not been done yet.”
It seems highly controversial to suggest that a patient could opt for experimental treatment in preference to an approved evidence-based therapy but Soria responds, “there is a difference between what is evidence based and what is scientifically or medically rational.” He gives the example of a patient with advanced melanoma that is not treatable with a BRAF inhibitor, and where ipilimumab has failed as first-line therapy. “What is my standard of care in second line? It is a lousy chemotherapy. What is my option in phase I? It is a PD1 inhibitor [a new class of immunotherapy currently showing great promise in melanoma]. Which do I choose? Anyone with a real mind would choose a PD1 because the likelihood of activity is 40%, while the likelihood of activity of standard chemotherapy is 10%. But that’s not evidence based, because the trial has not been done yet.”
“There is a difference between what is evidence based and what is scientifically or medically rational”
So if the choice is so obvious, why are medical oncologists not more eager to suggest joining a phase I trial before all standard lines of treatment are exhausted? “Because they don’t want to come clean about the fact that we do not know how best to treat them, and they do not know about mechanism of action of all these new drugs in phase I,” is Soria’s response. Doctors don’t like to admit they don’t know, “because it’s admitting our limitations.”
The truth, he adds, is that “In metastatic cancer, with the exception of hormone-dependent tumours or testicular cancer, the only certainty we have is that there is no cure, the patient will die. The question is, how can I delay that from happening while keeping quality of life acceptable? We need to think of all the potential anticancer approaches. Phase I is just one rational possibility. We need at least to discuss it with the patient.”
Rapid changes
Denis Lacombe, the headquarters director of the EORTC, Europe’s largest cancer clinical trials organisation, believes that access to trials will soon change substantially. The technologies behind next-generation sequencing are evolving so fast that generating detailed molecular data on patients’ tumours on a routine basis will soon be feasible and affordable. Medical oncologists will then have to decide what they do with the information. “If you are in a major cancer centre at the forefront of research, that is not a problem. But if you are in a middle-sized hospital in France, the UK, Germany, three or four years down the line, when you have next generation sequencing coming to you, what are you going to do for your patients? Data interpretation services will be critically needed. So there are plenty of changes that I’m sure the average medical oncology community have not anticipated, and they will come very fast.”
At the same time, drug development is becoming more targeted, says Lacombe, homing in on subpopulations of patients where the drug is likely to show the sort of major benefit now being demanded by payers – subpopulations like the 40–60% of melanoma patients with the BRAF mutation, or the circa 5% of patients with non-small-cell lung cancer who have a mutated ALK gene. If patients know they may be eligible for a trial that homes in on people like them, they have a huge incentive to join. Once, that might have meant looking for a large phase III; today, however, Lacombe disputes whether the concept of phase I, II and III trials is even meaningful any more, “We believe we should ban this terminology, because there is not such a firewall any longer between phase I, phase II and phase III.” The EORTC, he says, now advocates talking in terms of early trials, “designed to learn” and later trials, “designed to conclude”.
Early-phase trials now recruit patients in far greater numbers, to enable researchers to explore the drug in sufficient detail to learn who benefits, who is resistant, the best dose and schedule of administration, the most effective disease stage and the impact of combining it with other agents. A case in point is the PD1 inhibitor pembrolizumab, which recently hit the headlines at ASCO – Merck claims 1000 patients were involved in the phase I. Meanwhile, phase III trials are shrinking in size, says Lacombe, because such trials now have to demonstrate larger differences, and therefore require fewer patients to show statistical significance.
He points out that the early phases of a trial also take longer than they used to – and this is another good reason for patients to seek early access.
The challenge for the cancer research community, says Lacombe, is to ensure that all the data generated from earlyphase trials are used to greatest effect to deliver highly effective new drugs and treatment regimens. “The regulators are telling us that it is chaotic out there. You see all these companies developing new agents based on different technologies, even for the same biomarker and the same class of agent.” The challenge for patients and their doctors, may be locating relevant trials now that the whole picture is becoming so fragmented.
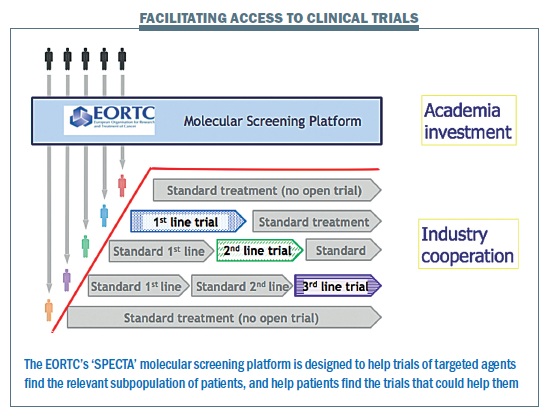
EORTC hopes that its new SPECTA platform – Screening Patients for Efficient Clinical Trials Access – may contribute to a solution to both problems, helping keep as much data in the public “precompetitive” domain as possible, and helping direct patients in a timely manner towards the most relevant trials. This initiative was outlined in detail by EORTC President Roger Stupp in the May–June 2014 issue of Cancer World. The idea is to work with doctors, hospitals and patient advocacy groups to ensure that newly diagnosed patients are asked for biological samples as early as possible, which would be sent to a central lab so their tumour can be subtyped and categorised at a molecular level. If the patient’s cancer recurs after standard treatment, they can then be offered a trial for second- or third-line treatment in the event that there is something available that fits their characteristics.
The intention, says Lacombe, is to “take the trial to the patient”. He believes it has the potential to transform patient access to relevant clinical trials.
“Patients screened at diagnosis could be offered access to any relevant trial if their cancer recurs”
 Patients’ strategies
Patients’ strategies
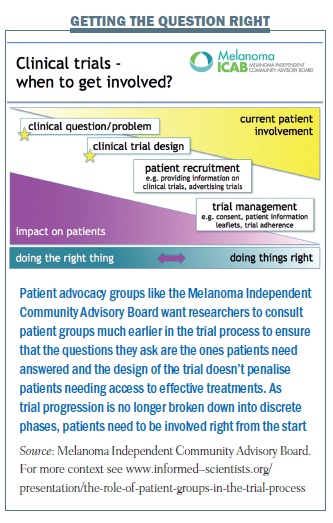
Not everyone is convinced, however, that channelling patients into earlier trials is necessarily in their best interests – or in the best interest of medical progress. Among the more vocal sceptics is Bettina Ryll, a medical doctor turned researcher with a PhD in molecular biology, whose husband Peter was diagnosed three years ago with an advanced aggressive melanoma, which eventually killed him. Together with other patients and advocates, they founded m-icab – the Melanoma Independent Community Advisory Board – which includes among its aims “aligning industry and investigators’ research goals with the best interests of those personally receiving treatment”.
 Ryll identifies with Soria’s chess analogy from her family experience, but questions the rationale for joining a trial of a drug whose benefit may be highly speculative if there are approved potentially effective alternatives. “As a melanoma patient, what you want is to live, so you are continually optimising your way through the system. Everything can change in an instant, for example with the approval of a new drug, the opening of an expanded access programme or a reimbursement decision. So if, for instance, we already have a PD1 on the market, which has shown unsurpassed overall survival benefit, why should a patient go for something highly speculative instead? After Peter’s diagnosis, we took one step at a time, looking each time for the best option available. And once we ran out of that option we started evaluating again. What is the best available therapy now? Then you go further down the line… and at some point there is nothing else out there.”
Ryll identifies with Soria’s chess analogy from her family experience, but questions the rationale for joining a trial of a drug whose benefit may be highly speculative if there are approved potentially effective alternatives. “As a melanoma patient, what you want is to live, so you are continually optimising your way through the system. Everything can change in an instant, for example with the approval of a new drug, the opening of an expanded access programme or a reimbursement decision. So if, for instance, we already have a PD1 on the market, which has shown unsurpassed overall survival benefit, why should a patient go for something highly speculative instead? After Peter’s diagnosis, we took one step at a time, looking each time for the best option available. And once we ran out of that option we started evaluating again. What is the best available therapy now? Then you go further down the line… and at some point there is nothing else out there.”
She is not in favour of a blanket message to encourage doctors to think about phase I trials for patients before standard treatment options have been exhausted. “We know that there are good phase I studies and not so good phase I studies. You have some researchers who believe so strongly in their research that they can’t wait to test it in patients. But even if there is a good rationale there is no guarantee it will work. And doctors have a lot of clout with patients. So I don’t think this is something that we should encourage indiscriminately.
“If you already have something you know works well, why should you opt for something highly speculative?”
“At the same time, if there is a phase I for a combination therapy, where each component drug is known to work very well, and early trials combining drugs with similar mechanisms have shown impressive results, then the rationale is sound. Joining that phase I might actually be a very good thing to do. There is no ‘one size fits all’, which is why I think it is dangerous to generalise.”
For patients trying to ‘optimise their way through the system’, the real problem, Ryll believes, is that when the best options are only available on a trial, patients want to join those trials when there is clear evidence of benefit – but to do so they must run the risk that they will be randomised to an arm that is known to be inferior. This she feels is a far more important issue.
While it is essential to learn as much as possible about any new drug and the best way to use it, and to follow results for benefit and toxicity over the longer term and with ‘real patients’, the question that really matters to patients like Peter is: “Will I do better on this agent than on other available alternatives?” And the answer to that is often known with some confidence at a relatively early stage.
This takes us back to Soria’s statement that anyone with advanced melanoma and no BRAF mutation should choose a phase 1 PD1 inhibitor over an approved standard chemo-therapy. Yet Merck’s PD1 inhibitor pembrolizumab is currently being trialled against a comparator arm consisting of “lousy” dacarbazine or alternative chemotherapies with no greater evidence of benefit (NCT01704287, clinicaltrials.gov). Ryll wants to know, what new knowledge is gained by letting more patients die on dacarbazine. “My pathology textbooks from years ago were already stating that melanoma does not respond to chemotherapy!” she says.
Ryll has some insight into how it feels to be randomised to such a control arm. She and Peter spent one of the worst weeks of their life together waiting to hear whether he had been randomised to GSK’s novel MEK inhibitor – known today as Teflinar – or to “lousy” dacarbazine after he had met the inclusion criteria for the trial. He hit lucky. Ryll describes the impact of the treatment as “almost miraculous”: Before the MEK inhibitor, the tumour that had started under the right arm had already encased his elbow joint, so he could barely use it to feed or dress himself, and the disease was progressing so rapidly they came to dread going to bed at night for fear of the visible change the following morning. And afterwards? Ryll answers by showing a photograph of Peter rowing across a lake with their children after exactly one month on therapy.
The principle of equipoise
At the time that Peter entered the phase III trial, says Ryll, there was not the slightest doubt that the MEK inhibitor worked better than dacarbazine, the ‘standard of care’. She believes that clinical trials in which it is known from the outset that one arm is clearly superior to the other are inhumane and unethical as they violate the principle of equipoise – the principle of not knowingly exposing patients to inferior therapies. “In melanoma, clinical trials have effectively become treatment and the best chance for survival, so patients’ desperation is used to fill unethical trials – this is no way to do medical research,” she says.
Time, resources – and patients’ lives – are being wasted merely to increase the certainty with which we already know something, argues Ryll. Worse still, she adds, the questions are always posed in a way that is designed to deliver that proof. “They stack the design in a way that they know there will be a difference coming out. So what’s the point of doing it? To produce nice statistical values? Well the statistics are there to serve patients not the other way around.”
“Time, resources – and patients’ lives – are being wasted merely to increase the certainty of what is already known”
Progress in delivering effective cancer treatments would be better served, Ryll believes, if the whole effort were concentrated on answering questions of genuine uncertainty – how can we make this work better, longer, control the side-effects? She quotes enthusiastically from a 2009 Lancet paper (vol 374, pp 86–89), written by Iain Chalmers of the James Lind initiative and Paul Glaziou of Oxford University’s Centre for Evidence-Based Medicine, showing that up to 85% of medical research funding is wasted – much of it because researchers are failing to ask the questions that patients want answered. Ryll says she has never been able to find anyone who defends these trials. She confronts the pharmaceutical companies, they blame the regulators. She confronts the regulators, they blame the health technology assessment bodies. “But when I question [the HTA bodies], they say ‘It’s not us. We don’t want these trials’. Everywhere I go, everyone points the fingers at the others.”
This gives her hope that there may be an opportunity for a new approach, and she points out that companies, regulators and HTA bodies have been discussing more efficient ways of working for many years. Ryll feels that patient advocacy groups like m-icab have an important role to play in making it happen. “We are the ones who are dying, so we are the ones who are motivated to change things and can push for it.”






Leave a Reply