Focusing on the UK situation, Willie Hamilton and colleagues investigate why speeding up diagnosis of symptomatic cancers may be important, how to achieve it, who to focus on and where, and finally how much such strategies could cost/save in economic terms.
This is an abridged version of Willie Hamilton et al. (2016) Improving early diagnosis of symptomatic cancer. Nat Rev Clin Oncol 13: 740–749, doi:10.1038/nrclinonc.2016.109. It was edited by Janet Fricker and is published with permission © Macmillan Publishers Ltd.
In all developed countries, much time, effort and finance is spent on cancer diagnosis, on the expectation that this approach brings clinical benefits. Before reviewing the evidence regarding how the diagnosis of symptomatic cancer can be improved, it is important to look at why the diagnosis of symptomatic cancer is necessary and should be improved. Only by being explicit about what we are hoping to achieve can we design services to meet our needs optimally.
Benefits of more rapid diagnosis
Types of evidence
 Few randomised controlled trials have investigated whether speeding up symptomatic cancer diagnosis improves patient outcomes, as it is hard to get ethical approval for trials where one group has delayed diagnosis. Trials comparing different diagnostic modalities have, however, been performed. The SIGGAR trial, for instance, compared the effectiveness of CT colonography versus colonoscopy for colorectal cancer symptoms (Lancet 2013, 381:1194–202).
Few randomised controlled trials have investigated whether speeding up symptomatic cancer diagnosis improves patient outcomes, as it is hard to get ethical approval for trials where one group has delayed diagnosis. Trials comparing different diagnostic modalities have, however, been performed. The SIGGAR trial, for instance, compared the effectiveness of CT colonography versus colonoscopy for colorectal cancer symptoms (Lancet 2013, 381:1194–202).
An alternative has been to perform trials of cancer diagnostics (promoting earlier presentation of potential symptoms) in community care settings. Examples include computerised decision-support tools in primary care cancer diagnosis (Trials 2016, 17:184) and lower symptomatic thresholds for urgent chest radiography (Trials 2013, 14:405). However, no trial has included sufficiently large cohorts to address whether speeding up cancer diagnosis in primary care benefits mortality or morbidity.
Survival and diagnostic activity
Survival benefit provides the main rationale for speeding up cancer diagnosis. Among European countries with a higher income, the UK and Denmark regularly appear at the bottom of tables ranking cancer survival (Lancet Oncol 2013, 15: 23–34). Poorer outcomes relative to countries at a similar level of economic development are considered to arise from differences in availability of, and willingness to use, cancer diagnostic investigations, augmented by English patients being less willing to seek medical care. One study reported inverse relationships between cancer survival and degrees of separation of primary care from specialist care, where specialist care requires referral from primary care (Br J Gen Pract 2011, 61:512–13). The association could be accounted for by unwillingness of ‘gate-keeper’ GPs to test for cancer when risks are small [see also ‘Should I refer this patient? p 44]. An ‘international vignette study’ asking GPs from 12 different geographical areas across three continents about fictitious patients revealed highly significant relationships between willingness to investigate cancer and national cancer survival (P<0.05 for four of five scenarios tested; BMJ Open 2015, 5:e007212).
 An English study showed that patients undergoing upper gastro-intestinal endoscopy at general practices that ranked in the top third for endoscopy rates had better overall survival (P<0.001) and fewer emergency admissions (P<0.001) than patients who were investigated at general practices ranking in the bottom third (Gut 2014, 63:250–61).
An English study showed that patients undergoing upper gastro-intestinal endoscopy at general practices that ranked in the top third for endoscopy rates had better overall survival (P<0.001) and fewer emergency admissions (P<0.001) than patients who were investigated at general practices ranking in the bottom third (Gut 2014, 63:250–61).
Considerable variations exist in use of cancer diagnostics in the UK. For example, in 2012–13, a 3.6-fold difference in CT use was observed between primary care trusts with the highest and those with the lowest CT use. Disparities also exist in terms of referral, with a three-fold difference between practices in the lowest and highest deciles for referral rate. In an English study (including 8,049 practices with 215,284 patients) cancer patients from general practices with lowest use of urgent cancer referral pathways showed excess mortality compared with intermediate use (HR=1.07; BMJ 2015, 351:h5102).
Findings from observational studies support the hypothesis that increased use of cancer diagnostics improves survival. This underpins the recommendation made by England’s Independent Cancer Taskforce that, by 2020, 95% of GP referrals for cancer testing should receive a definitive investigation and results within four weeks. Nevertheless, patients with one of six common cancers offered initial primary care diagnostic testing had a median time to referral of 16 days compared to zero days for those not offered primary care investigations (Br J Cancer 2015, 112:676–87). If suspected cancer investigations are used by GPs, diagnostic services need to be more responsive.
Time to diagnosis and survival
Time to diagnosis incorporates three elements: patient interval (beginning when bodily change is detected); primary care interval (beginning at first presentation to primary care); and secondary care interval (beginning with specialist referral). The diagnostic interval is the sum of the last two elements.
In a landmark systematic review of 87 breast cancer studies, clear relationships for worse survival were found for patients with delays of three months or more compared to shorter delays
(OR=1.47; Lancet 1999, 353:2155–62).
For colorectal cancer, diagnostic interval and survival studies reveal J-shaped curves. In patients presenting with symptoms suggestive of cancer or any other serious illness, the risk of dying within three years decreased with diagnostic intervals up to five weeks and then increased (Br J Cancer 2011, 104:934–40). The explanation suggested for the poorer survival among patients diagnosed very rapidly is that these patients will be the ones who present with the most aggressive disease with obvious symptoms, or who present as emergencies.
Morbidity and time to diagnosis
Reduced morbidity and improved symptom relief are possible benefits for quicker diagnosis. A study of 263 patients in Denmark showed significant associations between reported psychological distress and time to diagnosis (P<0.005; Anticancer Res 1996, 16:995–99). Another study among patients with colorectal cancer found no association between symptom duration and satisfaction with care (Can Fam Physician 2012, 58:e495–e501). A third study, in endometrial and ovarian cancer, revealed that total diagnostic intervals negatively correlate with quality of life (Qual Life Res 2012, 21:1519–25).
 Separating distress of diagnosis from additional anxiety from diagnostic delays is difficult. Initial distress resulting from the discovery of a symptom of breast cancer (measured on an emotional distress scale) negatively correlates with delays in presentation to healthcare systems (P=0.01; Prev Med 2003, 36:374–8). Associations may be complicated by a tendency for clinicians to investigate patients with anxiety or depression less rapidly.
Separating distress of diagnosis from additional anxiety from diagnostic delays is difficult. Initial distress resulting from the discovery of a symptom of breast cancer (measured on an emotional distress scale) negatively correlates with delays in presentation to healthcare systems (P=0.01; Prev Med 2003, 36:374–8). Associations may be complicated by a tendency for clinicians to investigate patients with anxiety or depression less rapidly.
Achieving quicker diagnosis
Pre-presentation factors
For most cancers, the time between first detection of potential symptoms by the patient and subsequent presentation to healthcare systems represents the greatest proportion of total time to diagnosis (Br J Cancer 2005, 92:1959–70). One study showed patients with oropharyngeal and oesophageal cancers were most likely to present 15 days or more after noticing an initial symptom, while another showed patients with prostate and rectal cancer were most likely to delay consultations by three months or more.
To speed up diagnosis, it is essential to understand how patients recognise possible symptoms and the decisions they make regarding seeking help. Symptom appraisal and help-seeking are influenced by psychosocial and cultural contexts, including fear of stigma, cancer diagnosis and treatment, and a belief in fatalism, as well as practical barriers to help seeking, such as a lack of access to health care, and sufficient time / transport to attend consultations.
Symptom awareness campaigns
Public campaigns raising symptom awareness might educate and empower people to hasten earlier presentation (Br J Cancer 2009, 101:S31–9). For example, between 2011 and 2012, Public Health England’s ‘Be Clear on Cancer’ campaigns increased attendance for lung cancer symptoms by 29% and bowel [colorectal] cancer symptoms by 63%. Notably, the percentage of lung cancers diagnosed at stage I (amenable to surgical resection) rose from 14.1% before the campaign to 17.3% after (P<0.001).
Cancer awareness campaigns need to address the health literacy level of their target audience, with lower health literacy strongly associated with disadvantaged socioeconomic and ethnic minority groups (BMC Health Serv Res 2008, 8:49).
Few studies of interventions specifically targeting individuals at increased risk of cancer have been conducted. However, a Scottish study on people at high risk of lung cancer (smokers and former smokers) provides preliminary evidence of altered consulting patterns following a single nurse consultation and provision of a symptom self-help manual (Br J Gen Pract 2013, 63:e47–54).
In primary care
 In most countries, symptomatic patients initially present to primary care, although some healthcare systems allow direct access to specialists. Clinicians must first think of cancer as a possibility and then decide whether testing is required. Some cancers are difficult to suspect, particularly when symptoms share common features with benign conditions. For example, although backache is the most frequent symptom of myeloma, only one in 1,000 adults reporting backache will turn out to have myeloma (Br J Gen Pract 2015, 65:e106–13). Such ‘difficult to diagnose’ cancers are characterised by three or more primary care visits before diagnosis. Consulting with the same clinician in the practice has only a very small effect on the rapidity of cancer diagnosis (Br J Gen Pract 2014, 65:e305–12).
In most countries, symptomatic patients initially present to primary care, although some healthcare systems allow direct access to specialists. Clinicians must first think of cancer as a possibility and then decide whether testing is required. Some cancers are difficult to suspect, particularly when symptoms share common features with benign conditions. For example, although backache is the most frequent symptom of myeloma, only one in 1,000 adults reporting backache will turn out to have myeloma (Br J Gen Pract 2015, 65:e106–13). Such ‘difficult to diagnose’ cancers are characterised by three or more primary care visits before diagnosis. Consulting with the same clinician in the practice has only a very small effect on the rapidity of cancer diagnosis (Br J Gen Pract 2014, 65:e305–12).
Clinical decision support
Insights into the epidemiology of primary care cancer symptoms, including estimates of positive predictive value, have enabled development of risk assessment tools predicting likelihood of cancer. Systematic reviews have indicated that clinical decision support improves physician performance and ordering of diagnostic tests.
The first evaluation of a risk assessment tool for patients with suspected lung or colorectal cancers found use increased two-week referral rates by 31% for lung cancer and 26% for colorectal cancers; and increased chest radiography by 4% and colonoscopy by 15%. It also resulted in increased cancer diagnoses by 37% for lung cancer and 76% for colorectal cancer (Br J Gen Pract 2013, 63:e30–6).
Risk algorithms include electronic tools interacting with patients’ individual clinical records, which involve doctors entering symptoms and calculating risk, with prompts to consider a cancer diagnosis when the combined features add up to a 2% or greater cancer risk. In an evaluation involving more than 500 UK general practices, use of tools increased urgent referrals by 19%. No studies have examined diagnostic utility of clinical judgement compared with evidence-based tools, although 2015 guidance from NICE [England’s National Institute for Health and Care Excellence] allows clinicians to override recommendations from decision support tools when there are good reasons to do so. More sophisticated artificial intelligence systems are currently in development and may be implemented in routine practice in the next few years (Br J Cancer 2015, 113:1645–50). In the latest revision of NICE guidance for suspected cancer, tools were not made the subject of recommendations as they had not been sufficiently studied.
Policy-driven initiatives
Early national intervention strategies designed to improve cancer outcomes prioritised treatment advances. By the early 2000s, however, some jurisdictions were seeking to speed up referrals of patients with high-risk symptoms, with the UK setting a two-week time frame.
The responsibility for cancer diagnosis could be extended beyond general practice to other providers of primary care, such as dentists and opticians, who identify oral and uveal cancers. At present, outside pilot studies, pharmacists have no access to diagnostic testing, and often have to refer symptomatic patients to GPs.
Patient and population aspects
Many cancer risk factors have been identified, but arguably risk factors other than age, sex and smoking should only be used in the selection of patients for screening, not for clinical assessment of symptomatic patients. Patients from ethnic minorities generally have worse cancer survival than prevalent majorities, but also experience more diagnostic delay (BMC Fam Pract 2013, 14:197).
NICE guidance on patient selection
In 2015 NICE guidance on selecting patients for cancer investigations was based on a cancer risk threshold of 3% or more, also allowing investigation for risks of less than 3% for children (who experience survival benefits long-term) and for widely available primary care tests, such as PSA testing. The decision to use a cancer risk threshold and the specific cut-off for referral were both contentious.
Alternatives include giving priority to cancers known to result in better patient outcomes from faster diagnosis and availability of diagnostic resources.
The decision to use positive predictive values (PPVs) for symptomatic cancer derived from primary care populations, as thresholds bring equity across cancers, and can be numerically integrated into general practice software, enabling automated calculations of risk based on symptoms.
PPVs derived from primary care, however, differ from those derived from referred populations, due to referral creating populations with substantially higher disease prevalence, which has led some specialists to express concerns that the recommendations fail to match their personal experience of cancer symptomatology (Lancet 2002, 360:2080).
Thresholds for cancer investigation
The final decision by NICE to recommend urgent investigation once cancer risk was 3% or more was a compromise between liberalisation of previous guidance and recognition that many people would opt for investigations on the basis of a risk as low as 1% (Lancet Oncol 2014, 15:232–40). Liberalisation to a 3% threshold should theoretically lead to expansion in testing. Between 2006 and 2015, imaging activity increased at 5.7% per year, and the number of urgent referrals made under the National Health Service’s (NHS’s) ‘two-week wait’ system passed one million referrals in 2012. At the same time as attempts have been made to speed up NHS cancer diagnosis, cancer survival in the UK has improved, narrowing the gap with other European countries (Br J Cancer 2015, 113:848–60).
Internationally, new referral pathways have been developed to support guidelines, enabling rapid assessment of patients with symptoms of concern. In the UK, Australia and Canada, patients referred using these pathways are seen by specialists within 14 days, while in Denmark patients are seen within four working days (Health Policy, 2012, 105:65–70).
Referral pathways have been criticised for restricting use to patients with specific – generally high-risk – symptoms (Br J Cancer 2014, 110:584–92), excluding around one-half of symptomatic patients. Consequently, in 2013 only 34% of all cancers in England were diagnosed as a result of referral pathways, resulting in recognition of a need for development of rapid assessment models for patients with less-specific or lower-risk symptoms (Br J Cancer 2015, 112: S65–9).
Influence of diagnostic programmes
Any symptom investigation programme, as well as identifying patients with non-malignant conditions, will also identify patients in whom the cancer was causing symptoms as well as patients with comorbidities where cancer was an unrelated finding (e.g. people with chronic obstructive pulmonary disease are at higher risk of lung cancer from past or current smoking).
Overdiagnosis
Overdiagnosis describes diagnosis in an asymptomatic person that does not result in a net benefit. While overdiagnosis is of more concern with screening programmes, expansion of diagnostic activity means there is also a possibility with symptomatic cancer.
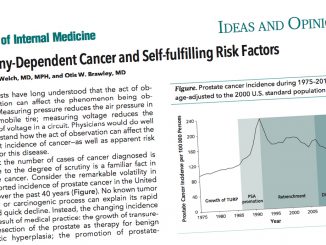
Currently, thyroid cancer, prostate cancer, and melanoma are the most likely to be overdiagnosed – e.g. thyroid cancer incidence rose 15-fold between 1993 and 2011 in South Korea, with no change in mortality observed (NEJM 2014, 371:1765–7). While evidence is limited, the authors suspect risks of overdiagnosis from expediting symptomatic diagnosis are small relative to possible benefits.
Health economics
Health economic analyses of costs versus benefits of expedited cancer diagnosis in symptomatic patients are less advanced than analyses of cancer screening performance. Diagnostic costs should include costs of negative results.
Comparisons of alternative diagnostic strategy costs are possible, with 2015 NICE guidance finding that faecal occult blood testing was the most effective approach for colorectal cancer (NICE 2015, http://www.nice.org.uk/guidance/NG12).
Data on cancer investigation performance in primary care populations, however, are rarely available, with little known about adverse events.
Estimating the benefits of more rapid cancer diagnosis is more difficult than estimating the costs of implementing such strategies. Establishing costs of treatment for various stages of cancer would be possible, with less advanced cancers cheaper to treat. Reporting stage shifts (if any) following cancer awareness campaigns would allow more informative health economic analysis.
Conclusions
In the UK, times to cancer diagnoses have fallen, as has the proportion of patients presenting with cancer as an emergency. Such progress is almost certainly a sign of improved diagnostics and have happened contemporaneously with liberalisation of the criteria for cancer investigation coupled with better identification of individuals most at risk. However, we do not yet know whether such attempts at early diagnosis are cost effective.
Key points
Very few randomised controlled trials have investigated whether speeding up the diagnosis of symptomatic cancer improves the outcomes of patients; however, observational evidence is indicative of clinical benefit for some patients.
Awareness campaigns often prompt earlier presentation of patients with cancer to the healthcare system, although the long-term effect of this earlier presentation is largely unknown.
Rapid access to specialist expertise, coupled with national guidance for selection of patients for investigation of possible cancer – and, subsequently, clinical decision support – might result in shorter times to diagnosis.
The UK National Institute of Health and Care Excellence recommend an explicit risk threshold of 3% for investigation of cancer in symptomatic patients; this liberalisation will influence the spectrum of patients seen by specialists.
The cost-effectiveness of initiatives to speed up diagnosis of symptomatic cancer is markedly under-researched.




Leave a Reply