Myeloma Patients Europe (MPE) has launched an overview of myeloma and AL amyloidosis treatment inequalities across Europe. The Myeloma Access Atlas, released 19 October, is a platform which has been developed to provide a ‘one-stop’ shop mapping national availability of myeloma and AL amyloidosis drugs in both EU and non-EU European countries, to provide data on comparative access.
“The Atlas is designed to allow patient advocates and advocacy organisations to see a snapshot of how their country ‘stacks-up’ in terms of access and to provide data and tools to inform national advocacy,” explains Ananda Plate, Chief Executive Officer of MPE. “National and comparative information on healthcare systems and drug access is incredibly important to understand health inequalities. Only with such baseline information can we start to analyse trends and identify the root causes and solutions to access challenges.”
To the best of their knowledge, says Plate, MPE is the first cancer patient organisation to have mapped access to drugs and drug combinations across Europe. A feat, she adds, made all the more challenging by the complexity of the myeloma treatment pathway.
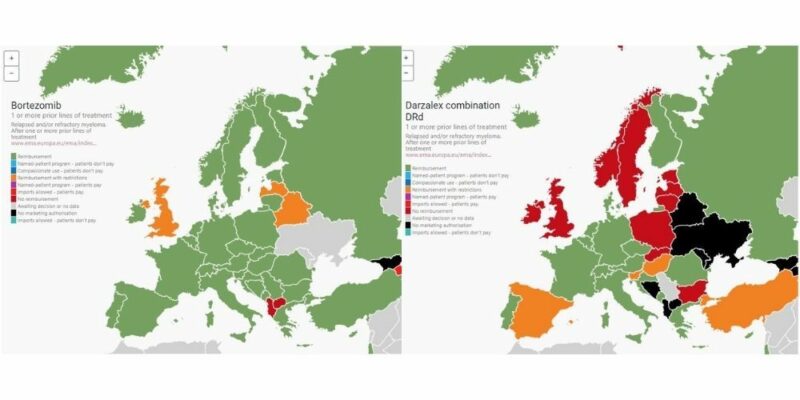
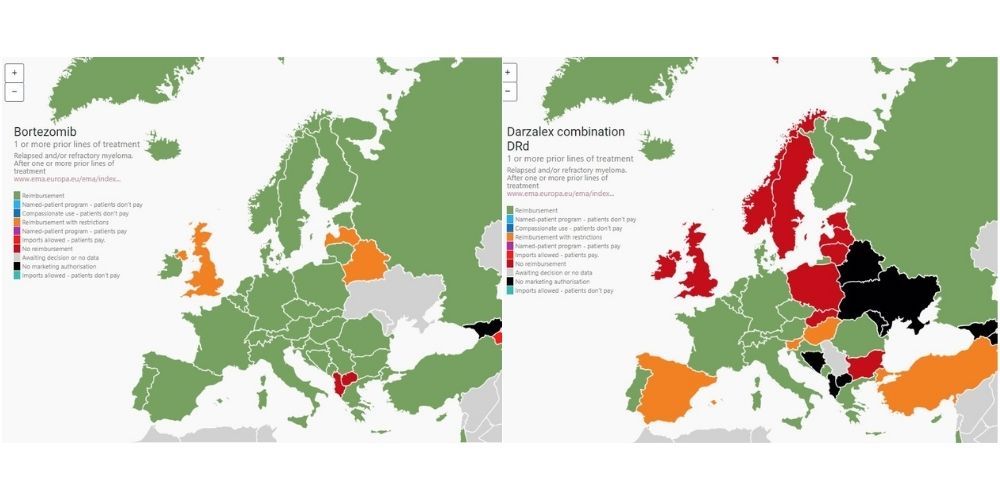
The Atlas includes access and reimbursement information on myeloma and AL amyloidosis drugs and drug combinations approved by the European Medicines Agency across geographical Europe. For this information, MPE reached out to multiple stakeholders. The process started by asking pharma companies in each country to provide information on access status of their myeloma drugs, with information then corroborated by MPE members and haematologists.
Access status, says Plate, proved altogether more complex than just stating whether drugs were reimbursed or not. The Atlas provides information across a range of detailed reimbursement categories, including whether they are funded by the healthcare system or available by programmes such as named patient programmes or via importation schemes.
The Atlas also gives comparative information on myeloma incidence and mortality for each country, alongside healthcare system performance data, such as GDP expenditure on health and out-of-pocket expenses. Development is an ongoing process, with MPE working to review health systems data contained in the Atlas on a quarterly basis.
Key findings from the first Atlas include:
- Many European countries do not meet recommendations outlined in the ESMO myeloma treatment guidelines.
- Triple and quadruple combinations approved for newly diagnosed patients face slow uptake across Europe. For example, evidence from the Atlas shows that the quadruple combinations involving daratumumab in the newly diagnosed setting are only available in a small number of countries. Balkan and Central Eastern European countries lag behind in access to triple combinations for patients who have had one to three prior lines of therapy.
- Access to maintenance therapy remains a big issue in Central and Eastern European countries.
- Macedonia and Bosnia face challenges in routine access to generic bortezomib (a backbone in myeloma treatment).
With such knowledge at their disposal, MPE is encouraging patient organisations to follow the Atlas Coaching Programme to develop strategies to overcome identified issues and challenges. The programme, available to complete online, asks advocacy organisations a range of targeted questions to help them identify their own advocacy priorities, goals, audiences, allies, messages and evaluation techniques. “All of which accumulate into a detailed strategy,” explains Kate Morgan, Head of Policy and Access at MPE. The MPE Access Team, she adds, is happy to assist organisations in developing plans to implement strategies once created or to provide one-to-one support on individual issues identified.
“As it currently stands, much of the information contained in the Atlas is focused on access to medicines, however we consider this to be only one part of the picture of access,” says Morgan. “Our strategy moving forward is to develop projects that gather evidence and develop solutions to wider aspects of access, such as optimal standards of diagnosis, treatment and supportive care at every stage of the journey.”