Posts by tag
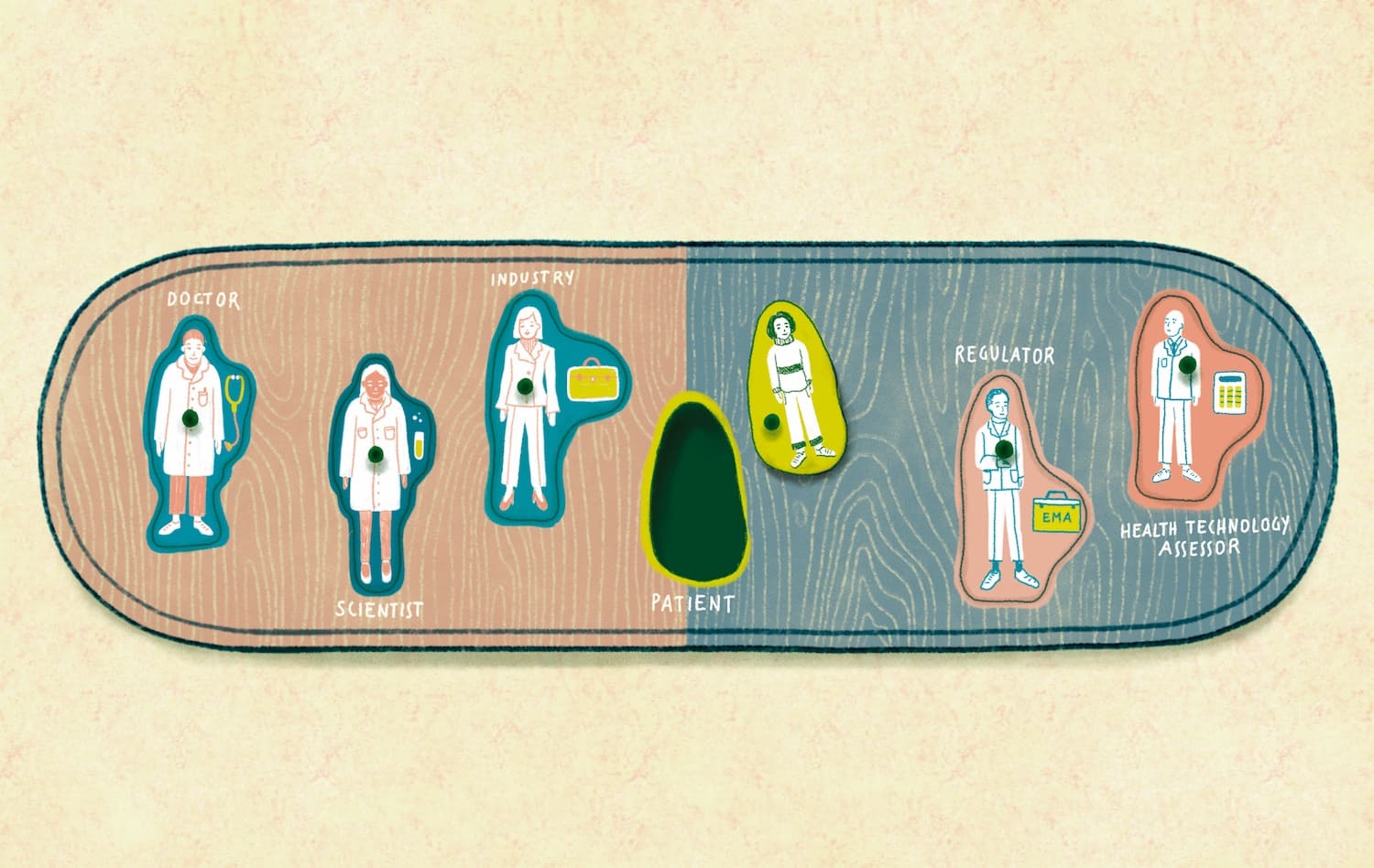
health technology assessment
Why Patient-Reported Outcomes are Rarely Used in Trials, and How We Change That
If drug developers design trials to measure how well their drug addresses the issues most important to the target patient community, we could expect to end up with better drugs. Integrating patient-reported outcomes into clinical trials is the way to…
Europe’s patient advocates skill up to better influence cancer care and research agendas
Patient advocates for a wide range of cancer communities across Europe spent four days at the start of July honing the skills they need to influence the policy, research and healthcare decisions that matter to them. This was the second…
EMA at 25: learning more from cancer patients
Responsibility for scientific evaluation, supervision and safety monitoring of medicines was done at the national level until 1995, when EU member states agreed to coordinate that work within the European Medicines Agency (EMA). A quarter of a century on, and…