Posts by tag
patient reported outcomes
Why Patient-Reported Outcomes are Rarely Used in Trials, and How We Change That
If drug developers design trials to measure how well their drug addresses the issues most important to the target patient community, we could expect to end up with better drugs. Integrating patient-reported outcomes into clinical trials is the way to…
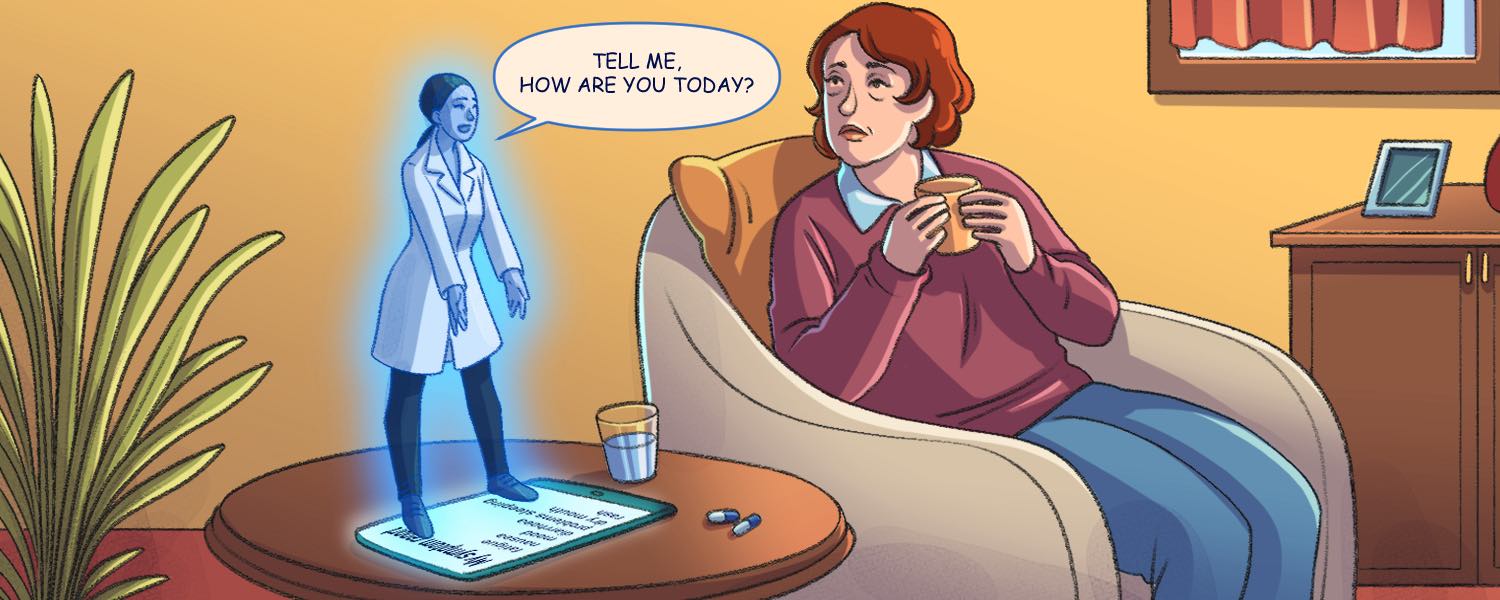
Regular symptom reporting can help your patients live better, and possibly longer
An older woman receiving treatment for breast cancer sits at home wondering whether to call her physician and go into the hospital. She’s on a new dose of a drug which she hasn’t had before and is struggling with nausea.…
A silver lining: Could changes forced by the pandemic point to better ways to conduct our clinical trials?
Pragmatic adjustments to trial protocols were seen to be essential during the Covid-19 pandemic to avoid trials being abandoned or delayed. Most changes involved reducing the requirements for travelling to centralised trials centres and reducing the level of reporting requirements.…