Androgen receptor activation exerts potent antitumour activity in oestrogen receptor positive breast cancer. The international study, published in Nature Medicine (18th January), has important therapeutic implications for women with metastatic breast cancers that are oestrogen receptor positive, including those who have become resistant to current forms of endocrine therapy.
“We provide compelling new experimental evidence that androgen receptor stimulating drugs can be more effective than existing (e.g. tamoxifen) or new (e.g. palbociclib) standard-of-care treatments and, in the case of the latter, can be combined to enhance growth inhibition,” says Wayne Tilley, the study’s principal investigator from the University of Adelaide, Australia.
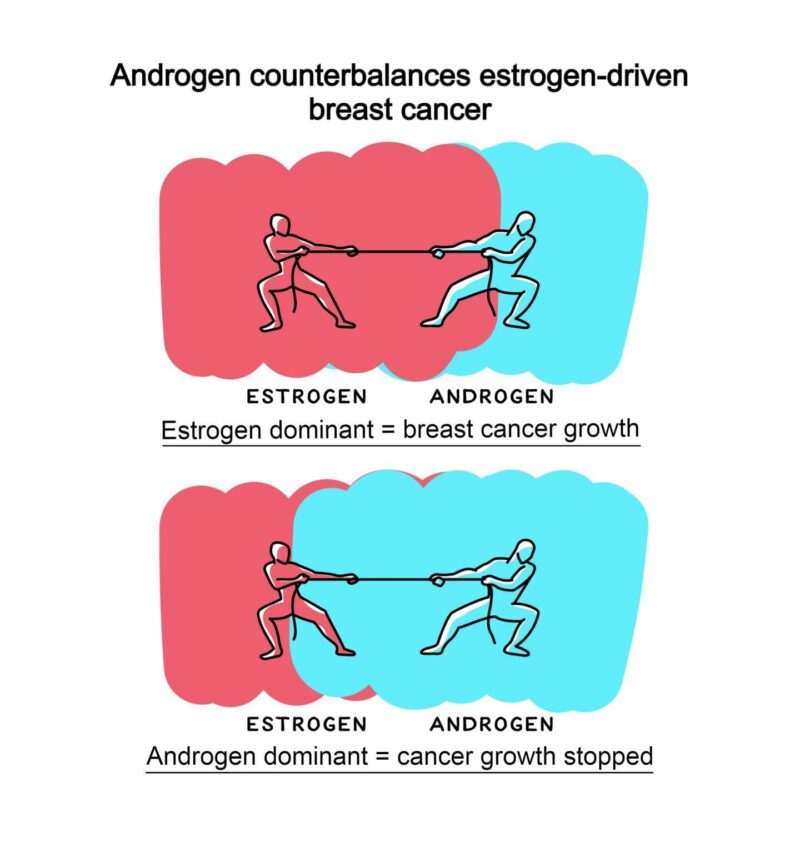
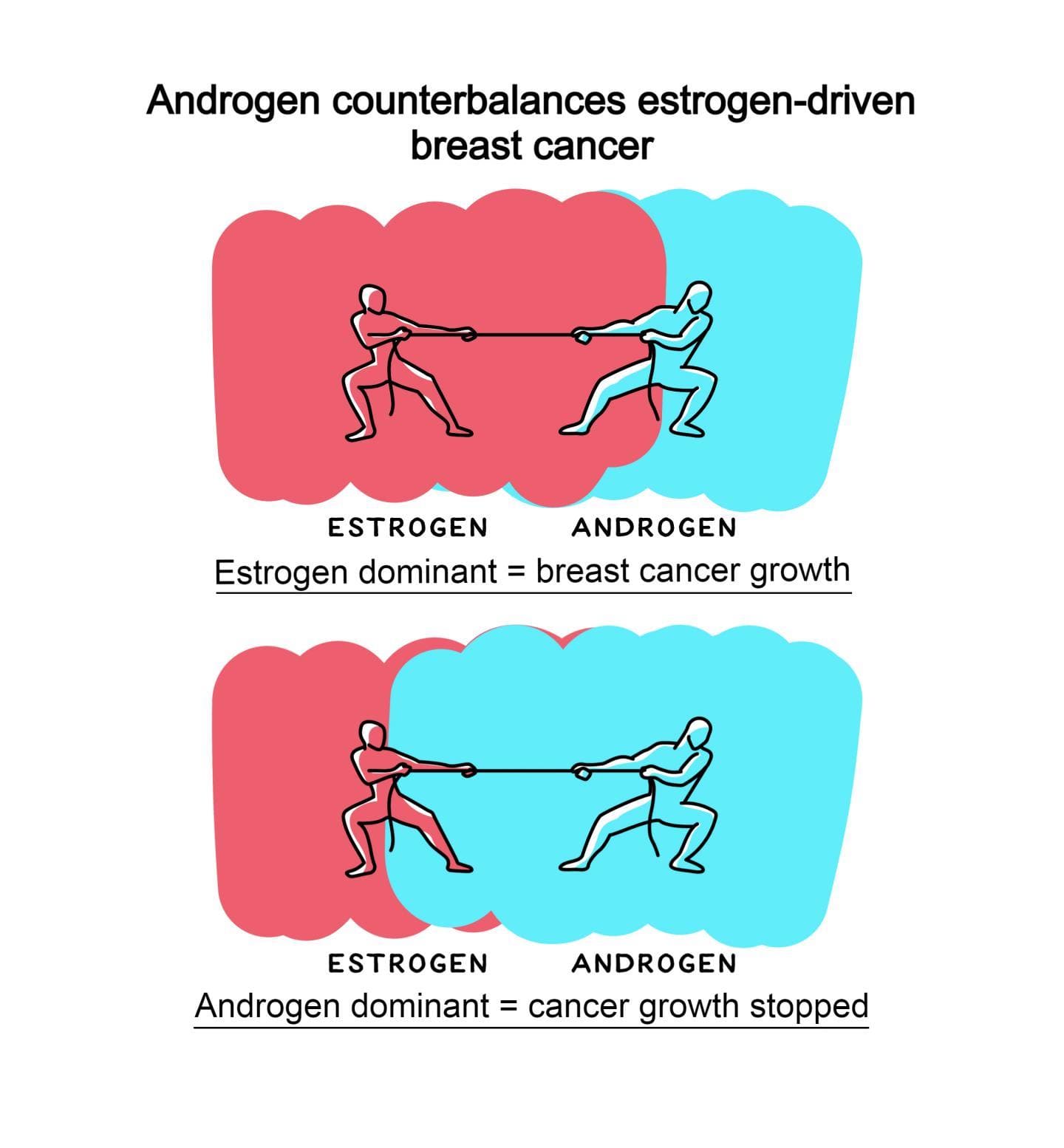
At puberty and throughout adult life it is known that oestrogen stimulates breast cancer growth and androgens inhibit it. Between the 1940s and 1980s androgenic compounds were used as breast cancer therapies, but despite clinical efficacy the approach fell from favour due to masculinising side effects (such as facial hair, acne, increase in haematocrit, or liver toxicity) and the advent of anti-oestrogenic endocrine therapies. With as many as 35% of women with oestrogen receptor positive breast cancer eventually becoming resistant to current hormone therapies, the need for alternative strategies has reawakened interest in androgen therapy. It is known that nearly all oestrogen receptor positive breast cancers also express androgen receptors and non-virilising selective androgen receptor modulators (SARMs) are now clinically available.
In the current study, Tilley and colleague used human breast cancer cell lines that were commercially available as well as tissue taken from breast cancer patients to demonstrate that direct activation of androgen receptors by both androgens and enobosarm (a drug with androgen receptor agonist activity) have potent antitumour activity in oestrogen receptor positive breast cancer. The team showed that oestrogen positive tumours treated with oestrogen alone had a higher proliferative index (ki67 positivity) compared to patient matched explants treated with oestrogen plus the potent natural androgen, 5 alpha dihydrotestosterone. Collectively, the data provides clear evidence of an anti-proliferative effect for androgens in clinical breast tissues exposed to oestrogen and suggests that androgen receptor signalling events that constrain normal breast growth can be reactivated by androgen receptor agonism.
“Taken together these data open up a potentially new chapter in the treatment of hormone driven breast cancer, one which is focused on stimulating the androgen receptor rather than blocking the female sex hormone receptor and it is hoped, given how they work, that they will have less side effects than treatments which block the oestrogen receptor,” says Carlo Palmieri, from the University of Liverpool, UK, who was one of the study investigators.
A phase II clinical study, presented by Palmieri at the 2020 San Antonio Breast Cancer meeting, evaluated the efficacy of enobosarm 9mg and 18 mg oral daily dosing in 136 heavily pre-treated women with oestrogen positive, HER2 negative breast cancer who had breast cancer progression. The results demonstrated a clinically meaningful clinical benefit rate of 32% and 29% in the 9 mg and 18 mg daily enobosarm cohorts respectively. Enobosarm was well tolerated and women reported improvements in quality of life measures like mobility, anxiety and pain discomfort. “Crucially, the results seen in the laboratory play out in the clinic, with enobosarm showing activity when given to women with advanced breast cancer. Importantly for a new drug it was also well tolerated and improved quality of life,” says Palmieri.
The international phase III ARTEST registration clinical trial evaluating enobosarm, in patients with androgen receptor and oestrogen receptor positive metastatic breast cancer who have failed a nonsteroidal aromatase inhibitor (anastrozole or letrozole), together with a CDK 4/6 inhibitor (e.g. palbociclib) is scheduled to start in the second quarter of 2021.