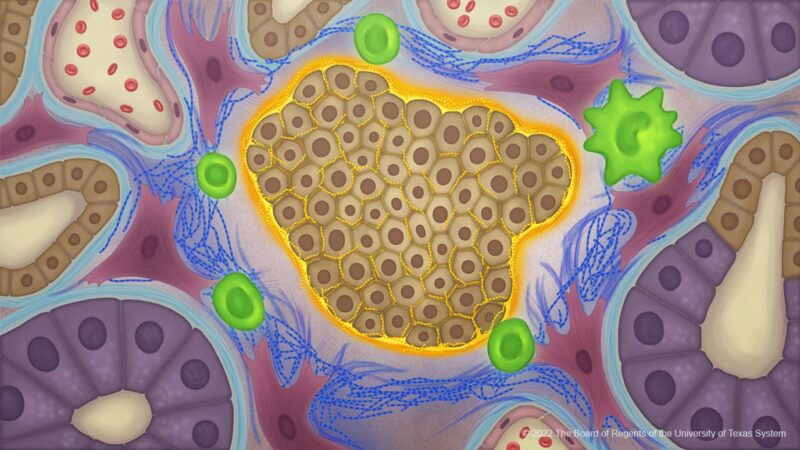
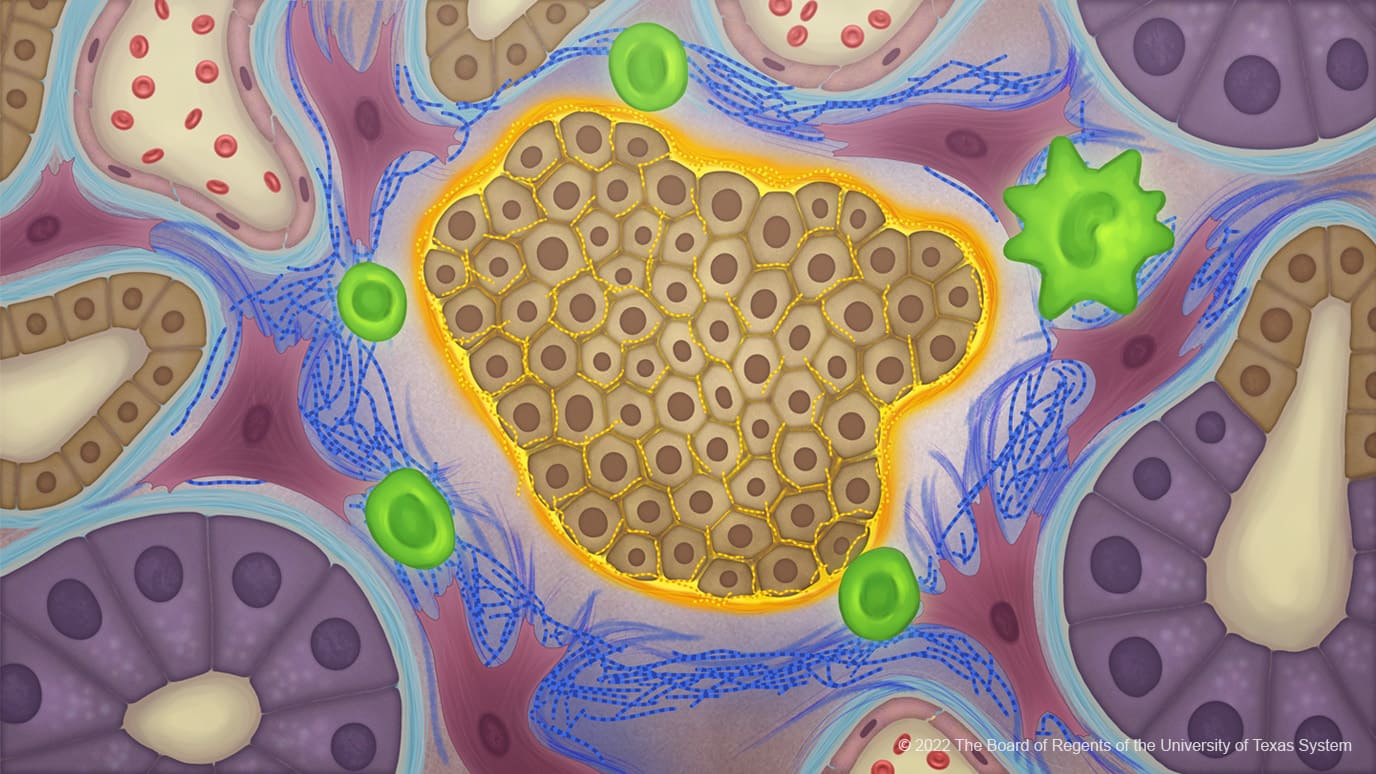
Cancer cells produce an atypical collagen in pancreatic cancer creating an extracellular matrix that influences the tumour microbiome to protect against immune responses. The study, published in Cancer Cell, July 21, suggests that such abnormal collagen could provide a new therapeutic target for a range of cancers.
“It turns out that the collagen made by pancreatic cancer cells is a unique structure that shields the cancer cells from the surrounding environment. It forms a protective cocoon or invisibility cloak that prevents the immune system from being able to see the cancer cells,” explains Raghu Kalluri, the senior author of the study from the James P. Allison Institute at The University of Texas MD Anderson Cancer Center, Houston. “This offers tremendous potential for the development of highly specific therapies.”
Collagen, the most abundant protein in the human body, is produced by fibroblasts and found mostly in bones, tendons and skin. During cancer development and growth, collagen is also known to accumulate in and around tumours, leading to the hypothesis that it may play a role in facilitating tumour growth, metastasis or drug resistance.
Collagen, in its normal form, represents a heterotrimer consisting of two α1 chains and one α2 chain which assemble together to form a triple-helix structure as part of the extracellular matrix.
Using western blot assays Kalluri and colleagues discovered that collagen from pancreatic cancer cell lines expressed only the α1 gene (COL1A1). Further investigations revealed that the α2 gene (COL1A2) had been silenced through epigenetic hypermethylation resulting in a cancer-specific collagen ‘homotrimer’ composed of three α1 chains.
The team undertook a genome-wide DNA methylation analysis that revealed prominent hypermethylation in the promoter regions of COL1A2 genes in multiple human pancreatic ductal adenocarcinoma cell lines. Consistently, COL1A2 hypermethylation status was inversely correlated with COL1A2 expression levels in pancreatic cancer. Treatment with demethylating agents, they discovered, increased expression of COL1A2 in both human and mouse pancreatic cells. “Collectively, the production of Col1 homo trimer consisting of only the Col1α1 chain is likely an overarching feature of both human and mouse pancreatic cancer cells,” write the authors.
Next, the team set about probing the role of cancer-Col1 in pancreatic cancer by generating genetically engineered mouse models (GEMMs) where Col1 was deleted only in pancreatic cancer cells. At 53 days of age, the investigators found that mice with deleted Col1 in cancer cells had significantly lower pancreatic tumour burdens compared with age-matched control mice. Loss of the three α1 chains, they discovered, reduced cancer cell proliferation.
Furthermore, the investigators found knockout mice responded better to anti-PD1 immunotherapy, suggesting that targeting cancer-specific collagen could help boost the anti-tumour immune response.
Increased overall survival of mice with pancreatic cancer and was furthermore associated with reprogramming of the tumour microbiome, which showed increased Campylobacterales and decreased anaerobic Bacteroidales. Furthermore, the altered microbiome was associated with changes in intratumoral immune cell profile, including decreased myeloid-derived suppressor cells (MDSCs) and increased T cells, with putative benefit in restraining pancreatic cancer.
However, the effects were reversed by disrupting the microbiome with antibiotics, suggesting that cancer-specific collagen promotes cancer progression through enhancing a tumour promoting microbiome.
“In addition to pancreatic cancers we believe that head and neck, lung and colon cancers may also form this abnormal collagen. It looks like this abnormal collagen may be involved in cancers that are harder to treat, but we need to do more work to discover how wide spread the process is,” says Kalluri.
In a mouse study published last year in Cancer Cell, Kalluri and colleagues found when they genetically deleted collagen from cancer-associated fibroblasts (as opposed to cancer cells in the current study) that pancreatic cancer growth was accelerated and the overall survival of mice decreased. “Our current paper brings our work full circle showing the totality of what collagen does in cancer. Fibroblasts make collagen that is protective against the cancer; while cancer cells make collagen that facilitates cancer growth,” explains Kalluri.
Next Kalluri and colleagues plan to look for ways to exploit their collagen discoveries to identify new therapy targets. “Since the type of collagen found in pancreatic cancer cells is not present in other cells in the human body, we could develop engineered T cells against it. Furthermore, we have found that the abnormal collagen binds to a receptor on the surface of cancer cells, called alpha 3 beta 1 integrin, we could block with monoclonal antibodies,” explains Kalluri.
Image credit: Copyright used with the permission of The Board of Regents of the University od Texas System through The University of Texas MD Anderson Cancer Center.