According to Francesco Pignatti, Head of Oncology at the European Medicines Agency (EMA), the term ‘tumour agnostic’ is a misnomer. The definition of agnostic in ancient greek, he argues, is ‘lacking in knowledge’. But with these new approaches, it’s not that we don’t know, “it’s a situation where we have comprehensive evidence, so in a sense, it’s a very gnostic situation!” Nevertheless, ‘tumour agnostic’ (or ‘tissue agnostic’) is a term that has stuck to describe therapeutics that treat the molecularly targetable abnormalities that fuel cancers across multiple tumour types. This approach has the potential to completely change the way patients are treated, but there are questions about how our regulatory and health systems would need to adapt to this new paradigm.
In 2017 the US regulatory authority, the FDA, granted accelerated approval for the use of pembrolizumab (Keytruda), a programmed death receptor-1 (PD-1) inhibitor, for treating solid tumours that are either microsatellite instability-high (MSI-H) ‒ where the cancer cells have a high number of mutations within tracts of repetitive DNA known as microsatellites ‒ or DNA mismatch repair-deficient (dMMR) ‒ where the cells are unable to repair mistakes made during the division process, leading to accumulations of mutations. This represented an expansion from its previous approval for metastatic melanoma, metastatic non-small-cell lung cancer (NSCLC), head and neck cancers, and classical Hodgkin lymphoma. From five clinical trials, the drug was also judged effective for endometrial cancer, gastric cancer, pancreatic cancer and biliary cancers, where appropriate biomarkers were present.
The first FDA tumour-agnostic approval for a drug not already in use came in 2018, with the tyrosine kinase inhibitor larotrectinib (Vitrakvi). It was approved to treat any advanced solid tumour with mutations in the NTRK genes that drive tumorigenesis. The pivotal study showed an impressive 75%‒80% overall response rate in 12 different cancer types that all had NTRK fusions (Drilon A et al NEJM 2018). In August 2019 the FDA approved another tumour-agnostic drug, entrectinib (Rozlytrek), for treating metastatic solid tumours that have an NTRK gene fusion, where no alternative therapy exists, and metastatic NSCLC with fusions in the ROS1 gene. By 2019, the EMA had followed suit and granted larotrectinib its first tumour-agnostic approval.
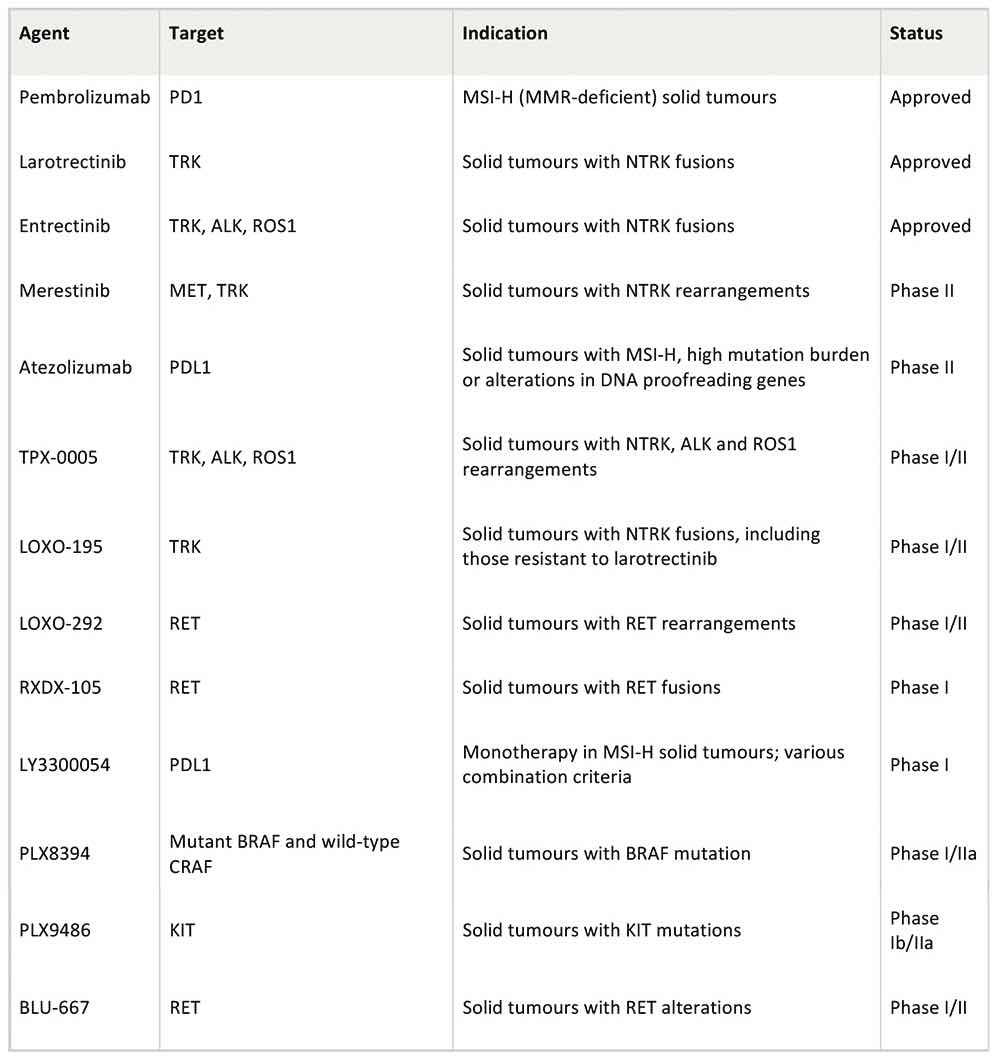
At least 10 further tumour-agnostic therapies are in development, based on a range of genetic mutations, including mutations in the RET gene, found in 2.21% of all cancers, and mutations in the neuregulin 1 gene (NRG1), which is found across solid tumours in lung, pancreas and breast tissue. But whilst tumour-agnostic approaches have attracted a lot of attention, it is still a niche area. “The amount of companies and developers who claim that they are pursuing such development for the time being is relatively small, and whilst we cannot foresee the future, many think that this is not going to be the predominant approach,” says Pignatti.
Clinical development ‒ basket trials
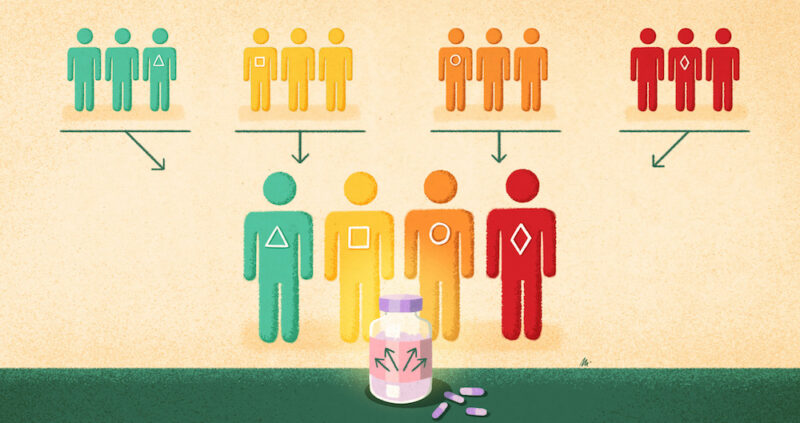
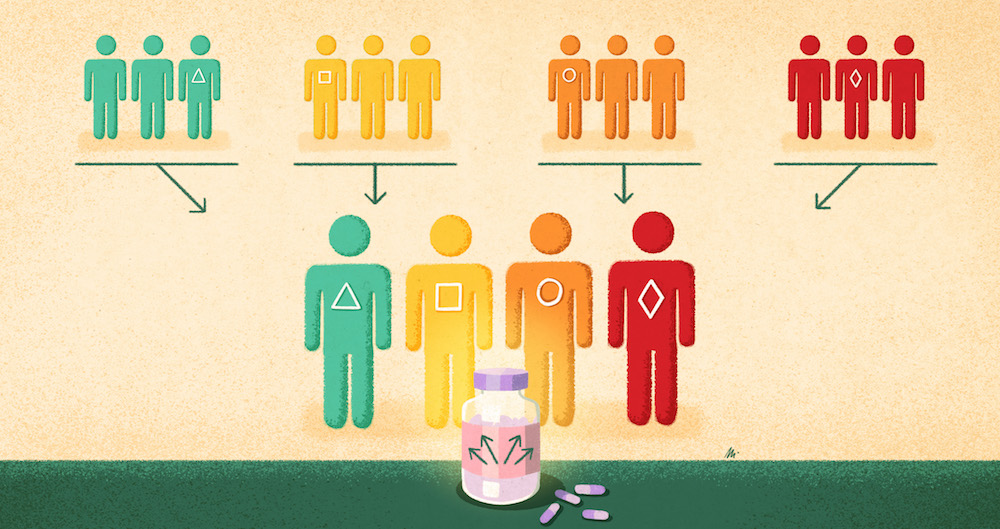
One of the innovations needed to develop tumour-agnostic drugs has been clinical trials that can span multiple histologies. These are known as basket trials (or sometimes bucket studies). They are currently done in multiple ways but all governed by an overarching master protocol, often with specific treatment ‘arms’ or ‘baskets’ for cancers of different origins. “I’ve seen in practice different examples… you can decide how independent the different sub-studies are. You can have one sub-study for breast cancer and another one for lung cancer, or have one single study where you pull them all together,” says statistician Olivier Collignon, from the Luxembourg Institute of Health and co-author of a study on statistical and regulatory perspectives on basket trials (Collignon O et al Clin Pharmacol Ther 2020). Another new type of trial, an umbrella trial, studies multiple therapies in different biomarker-matched patient subgroups with the same cancer histology.
Whilst these trials are currently more common in exploratory phase II settings, they have started moving into the regulatory setting. One of the first basket trials used for approval was for vemurafenib (Zelboraf), approved by the FDA in 2011 for treatment of melanoma based on the BRAF V600E genetic mutation. A basket trial also concluded that patients with the rare blood cancer Erdheim-Chester Disease who carried a BRAF mutation could also benefit from the drug (and approval for that indication was granted in 2017).
Large basket trials are becoming a key feature in oncology trials. “It allows [us] to be more efficient, but also [it allows] better partnerships,” says Denis Lacombe, Director General of the European Organisation for Research and Treatment of Cancer (EORTC) in Brussels. An example is the ‘Basket of Baskets’ trial run by Cancer Core ‒ a collaborative group of seven centres of excellence spread across France, Spain, Italy, Germany, Sweden, the Netherlands and the UK. The trial is currently testing the novel PD-L1 immunotherapy drug, atezolizumab. It aims to screen 1,000 patients over two years from patients treated at the seven centres, using a common molecular profiling platform to match patients to targeted therapies.The trial will also add other new experimental drugs from other pharmaceutical companies that target different genetic mutations (Brana I et al JCO 2019).
The EORTC has embarked on a biomarker-led umbrella trial called UPSTREAM, focused on head and neck cancer (carcinoma, squamous cell of head and neck). “There are multiple partnerships with several companies so that we can try to match each cohort of patients with the most probable drug to the benefit them,” says Lacombe.
“What we like in this type of trial is that it allows us to apply the concept of ‘leave no one behind’, so you try to offer as many solutions as possible”
One positive change being seen with tumour-agnostic approaches is the inclusion in clinical trials of patients with rare cancers. “Rare cancers have not been in the spotlight, they get less attention, and that [has been] one of the benefits of having the capacity to understand the biology of cancer,” says Lacombe. For example NTRK fusions are present in only 1% of solid tumours, but if histologies are looked at together, it makes a big enough market for drug development to be worthwhile ‒ hence the development of larotrectinib and entrectinib.
The evolving framework of clinical trials used to support oncology drug approval
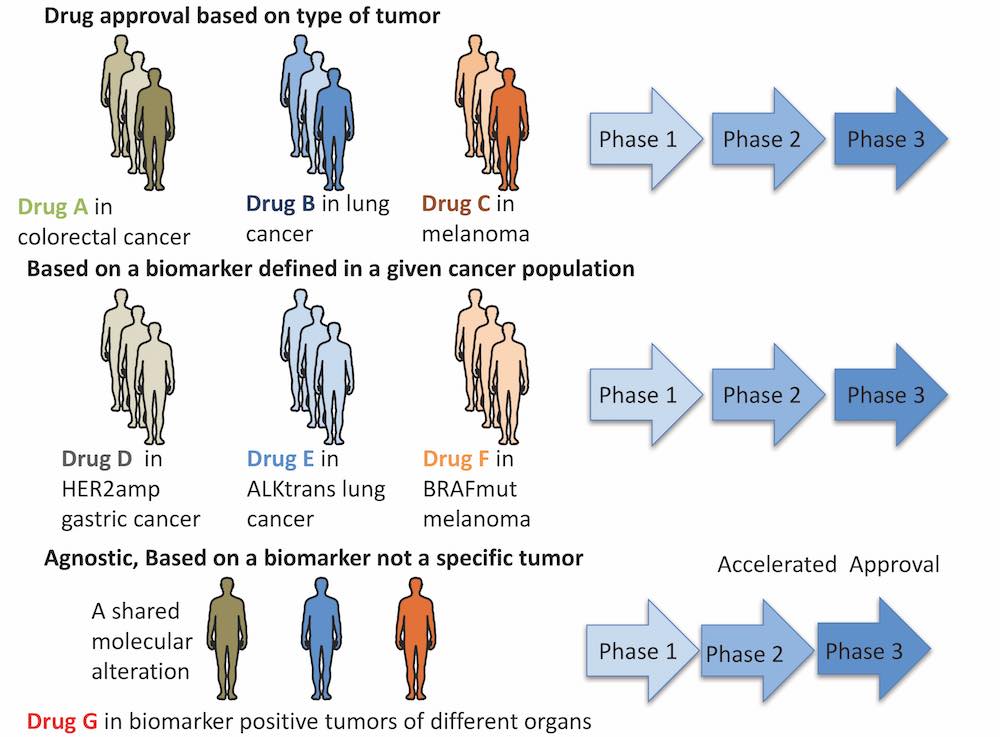
Uncertain evidence ‒ are the baskets too small?
But these new types of trials have led to some concerns. “If we are talking about trials designed to learn, for hypothesis generating and understanding the biology, I think that they may play a very important role, because they allow us to progress so rapidly,” says Lacombe, but he adds, “When it comes to trials to conclude ‒ to change practice ‒ that’s a little bit more difficult.”
The unease comes from the relatively small amounts of data that have been used in some tumour-agnostic basket trials. For example pembrolizumab was approved in the US based on five studies in 149 patients across 15 histologies, and larotrectinib was studied in three trials including 176 patients across 12 histologies ‒ significantly less data for each tumour type than would usually be expected. “We’ve seen drugs coming on the market earlier and earlier based on sometimes very small data sets. And it can be [an] innovation, but we have also seen a lot of examples where actually what was called innovation turned out not to be an innovation in terms of patient benefit,”says Lacombe.
The challenge for regulators is the added heterogeneity when different histologies are considered together. Basket trials include rare cancer types that share biomarkers with much more common cancers. Not only are the numbers of patients involved very different, but they may have widely different prognoses. “A 10% or 20% improvement in prostate cancer sounds very low to me. Whereas if you take a 20% improvement in multiple myeloma, or [another] more aggressive cancer, the figure is the same, but from the regulatory point of view, it doesn’t mean the same thing,” explains the statistician Collignon. And as cancers of different origins currently have very variable existing treatment options, it makes the benefit‒risk assessments for an entire group very difficult.
Pignatti says that using what were initially exploratory basket trials for regulatory approval “requires a rigorous and planned way to minimise statistical error.” One critical issue is controlling what are known as type 1 errors ‒ results falsely indicating that a therapy is effective, when it is not. The heterogeneity of basket trials increases this risk due to the multiplication of errors present in each sub-group and in comparisons across multiple treatment arms and multiple comparisons over time. Currently regulators mandate statistical errors must be less than 5%, and Collignon says basket trials are particularly prone to error inflations above this level.
The drugs approved on a tumour-agnostic basis so far have been given conditional approvals, which means they can be legally marketed if there is a reasonable expectation of effectiveness even if the data is not complete. The pharmaceutical company is then expected to carry out extensive follow-up studies. “There is more emphasis on post-marketing data generation… they’re expected to systematically collect data on efficacy and safety, or histologies that were considered to be less well represented at the time of approval,” says Pignatti.
The EMA does not currently have specific guidelines for the use of basket trials in tumour-agnostic therapies, but Pignatti says they are being developed. While it is likely that each case will differ and will need to be assessed on its own merits, it also seems likely that the traditional phases of clinical trials may start to change, with more emphasis on large basket trials designed to explore multiple cancers.
Uncertain value
“A final elephant in the room is the cost of such agnostic drugs,” says Roberto Salgado, a pathologist at the Breast Cancer Translational Research Laboratory at the Institut Jules Bordet, Brussels and GasthuisZusters Antwerpen (GZA) in Belgium. “Reimbursement agencies may not be willing to fund costly drugs, based on phase II trials, where the full solid cancer population would need to be tested [for the biomarker].” Pignatti agrees that assessing value could be a problem, given that many of the current tumour-agnostic drugs have been approved based on very little data on overall survival rates ‒ information critical for evaluating cost-effectiveness.
In April, England’s National Institute for Health and Care Excellence (NICE) and Germany’s Institute for Quality and Efficiency in Health Care (IQWiG) rejected larotrectinib (Vitrakvi), at £15,000 (almost €17,000) for a 30-day course, due to its uncertain cost-effectiveness, given the limitations of available data and the lack of any other similar drug with which to make comparisons. The drug could have been useful for an estimated 700 patients in England, and marketers Bayer claimed that the assessing authorities did not yet have the right methods to assess tumour-agnostic approaches.
Lacombe suggests one solution could be to scale costs to reflect this lack of data:
“The cost should be proportional to uncertainty and eventually be revisited one way or the other, when it is confirmed to be a true benefit to the patient and to society”
Diagnostics
The failure of larotrectinib to get through cost-effectiveness assessments contrasts with the enthusiasm shown by NHS England chief executive, Simon Stevens, who told a conference in 2019 that the NHS must be ready to fast-track tumour-agnostic therapies and prepare for the diagnostic testing that will be required to identify genomic mutations. Tumour-agnostic therapies will rely on European health services having the capacity for this testing. “National healthcare settings are still not equipped to fund or organise a systemic analysis of all solid cancers for genomic aberrations,” says breast pathologist Salgado.
France and the UK have prioritised the development of a next-generation sequencing infrastructure, but prioritisation and support for precision medicine diagnostics is still lacking, particularly for rarer mutations such as NTRK gene fusions. There is also little standardisation of approaches, says Salgado. “Not all laboratories use the same gene panel, which is also a prerequisite to test for specific genomic markers, meaning that a patient tested in centre X with panel Y, for potentially trial Z, may not be identified in another laboratory that uses another panel that does not contain that particular gene [biomarker].”
Another contentious issue is whether a particular diagnostic test should be developed to accompany a specific drug ‒ known as a companion diagnostic. “I’m not in favour of linking drugs to assays, as this creates an unfavourable context for [the] development of new assays,” says Salgado. “Why should a company promote another biomarker, which is easier to perform in laboratories and [may] be less expensive, if they have an assay approved with the drug?” From the patients’ perspective, he adds, “it makes sense to integrate gene panels with as many genes as possible, so that national cancer registries have a collection of the most important genomic events in solid and haematological tumours, and patients don’t have to fear that their tumour will be tested with suboptimal gene panels.”
Salgado also points out that the integration of new biomarkers into diagnostic panels is severely hampered by the fact that developments are driven by industry, with few academically developed biomarkers integrated in daily practice over the past decades. “To make this happen more frequently we need to collaborate with industry and regulatory [bodies] early on in trial design, to integrate new biomarkers in drug-driven clinical trials,” he says.
How tumour-agnostic can treatments be?
Practicalities aside, there are still also fundamental questions about the tumour-agnostic approach and how effective it will turn out to be. There are already several examples where a genetic marker turns out to have a different impact on the progression of a tumour, depending on its histology. Kinase inhibitors targeted at the BRAF oncogene, for example, have not been shown to be tumour agnostic. BRAF mutations are present in roughly 50% of melanomas and 10% of colorectal cancers, but only melanoma patients responded dramatically to BRAF inhibitors (Kopetz J et al JCO 2010).
From Genentech’s non-melanoma basket trial for the BRAF inhibitor vemurafenib in 2012, it was discovered that, in colorectal cancer, BRAF inhibition triggers the epidermal growth factor receptor (EGFR) signalling pathway that drives cancer proliferation ‒ a pathway not active in melanoma (Prahallad A et al. Nature 2012). More recently, the FDA approved a combination of BRAF inhibitors with EGFR inhibitors for treating metastatic colorectal cancers.
Differences were also found in a multi-histology basket study of the pan-HER kinase inhibitor neratinib (Nerlynx) from Puma Biotechnology, which targets both HER2 and HER3 receptors and is approved by the EMA for treating HER2-positive breast cancer. The study found clinical responses in patients with breast, cervical, biliary, salivary, and non-small-cell lung cancers, but not in those with bladder cancer and colorectal cancer.
Even before tumour agnostic approaches, there have always been differences in drug efficacy amongst patient sub-populations such as by age-group, gender, and general health status, and Pignatti suggests that, for regulators, histology may become just one more factor that needs to be considered. “Histology will be a question, but all the characteristics of a population will be looked at, and if they’re not homogeneous, you need to ask the question: Are there sub-populations where it can be shown that the drug doesn’t work? ‒ and then we will have to review our concept of a pan-histology efficacy to something which is a little bit more specific.”
“You need to ask: Are there sub-populations where the drug doesn’t work? ‒ then we will have to review our concept of a pan histology efficacy to something a little bit more specific”
For some clinicians the focus on tumour-agnostic therapies is unjustifiably overshadowing other areas of cancer treatment. The issue has sparked differences in opinion in the oncology community. An analysis by Vinay Prasad at the Oregon Health & Science University in Portland found that only about 9% of patients with metastatic cancer will be eligible for a genome-targeted drug, and just 5% will benefit from the therapy. He says the enthusiasm surrounding the promise of precision medicine needs to be tempered (Marquart J et al. JAMA Oncol 2018).
Others say that a growing number of patients will benefit as more tumour-agnostic therapies are approved. In a recent Science article, oncologist David Hyman reported that Memorial Sloan Kettering Cancer Centre, in New York, had tested tumours of more than 25,000 patients: 15% could already be matched to an FDA-approved drug; a further 10% could be matched to drugs in clinical trials, and a further 10‒15% to drugs currently in pre-clinical animal trials.
But is too much attention and funding given to these approaches at the expense of other important therapeutic avenues? “To some extent, I agree with this statement,” says Lacombe. “Not everything is about drugs. It’s also about improving our radiation oncology techniques. It’s bringing new technologies to patients. It’s also improving surgical approaches ‒ cancer is a very interdisciplinary field, it’s a disease that is treated by an interdisciplinary team, so we should look at the palette of treatments that we have.”
Clearly the picture will never be as simple as one drug for one biomarker regardless of the cancer type. Tumour-agnostic approaches represent the next step in precision medicine and our improved understanding of cancer biology. But it may be that they will continue to be the exception rather than the rule. “There remains an array of uncertainties and we have to understand, actually, why some patients are not going to benefit,” says Lacombe. “We should remain humble. We are making progress, but we should be conscious of our limits, and what we say out there to patients.”
Selected tumour-agnostic drugs in clinical development