Postmenopausal women with dense breast tissue compared to women of the same age who do not have dense tissue demonstrate upregulation of 124 proteins which may be responsible for creating pro-tumorigenic microenvironments. The study, published in the British Journal of Cancer, 31 October, found women with dense breast tissue had upregulation of 65 proteins linked to inflammation, 34 proteins involved in growth and/or metabolism, and 25 proteins involved in angiogenesis.
“Our data suggest that there is a major biological difference between dense and nondense breasts with a distinct profile of proteins related to inflammation, angiogenesis and growth factors and/ or metabolism in dense breasts,” write the authors. “All these events are included among the hallmarks of cancer and represent a pro-tumorigenic microenvironment, which may enhance the ability of atypical cells to invade and progress into clinically definitive cancer.”
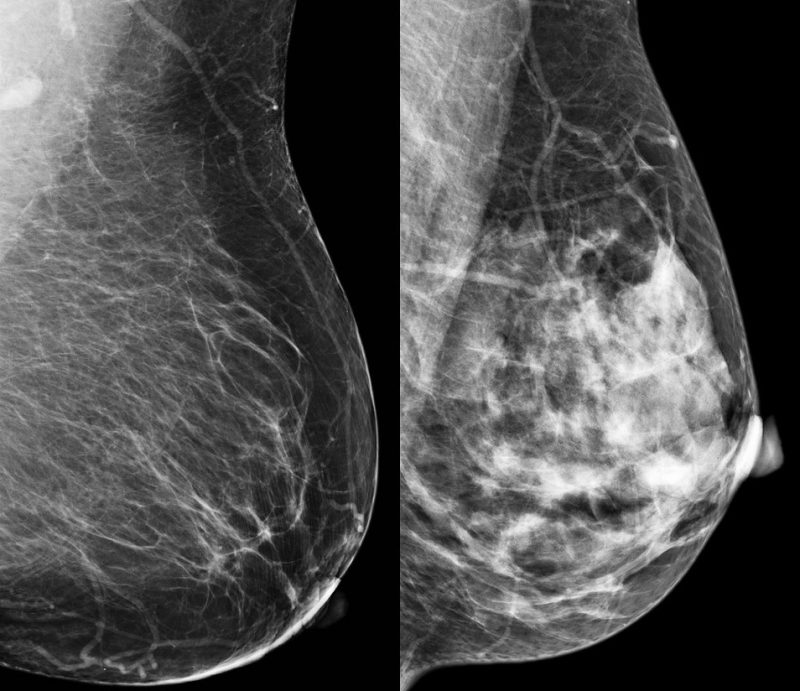
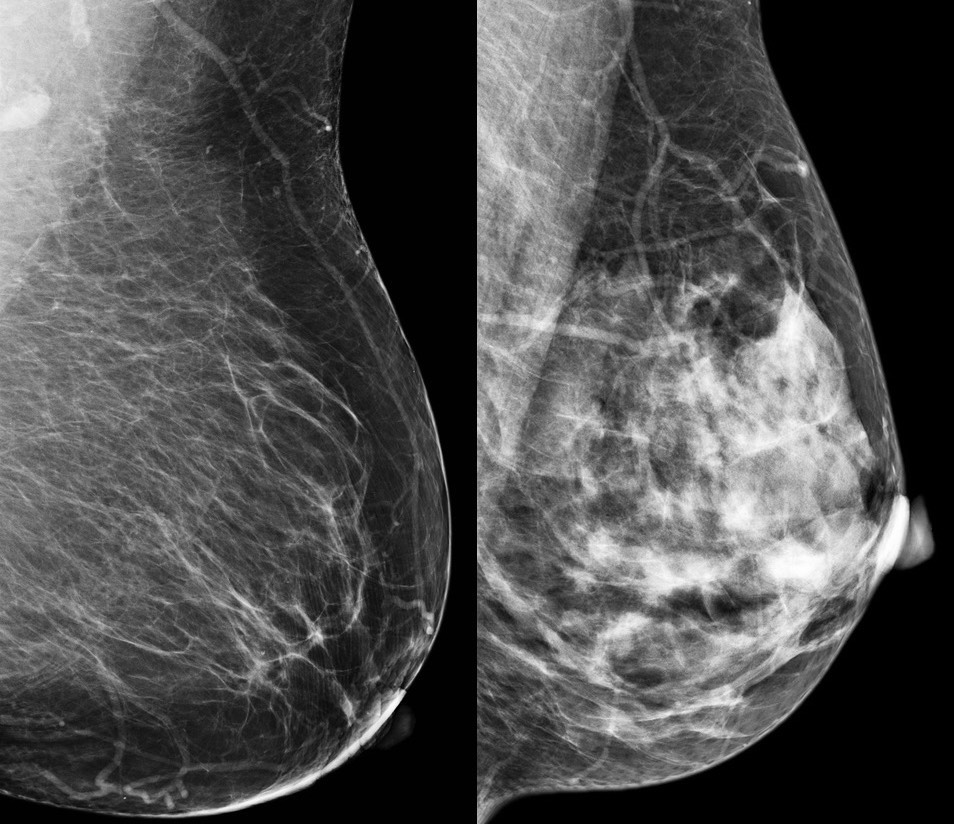
Epidemiological evidence shows that higher breast density is associated with increased risk of developing breast cancer. Women with dense breast disease face the dual problem of dense breasts masking detection of cancer (fibrous tissue, like cancer, shows up white) as well as a four-to six-fold increased risk of developing cancer. However, the difficulty in detecting tumours does not fully explain the higher risk of cancer in women with dense breast tissue.
In the current study, Charlotta Dabrosin, from Linköping University, Sweden, and colleagues, set out to quantify selected imaging biomarkers that specifically correlated with regional microstructural tissue properties in healthy postmenopausal women with dense or nondense breast tissue.
Between 2014 and 2015, 44 postmenopausal women ≥55 years of age were recruited from the screening mammography programme at Linköping University Hospital. Regular mammograms were assessed by one experienced observer according to the Breast Imaging Reporting and Data System (BI-RADS) density scale, with breast densities categorised as either BI-RADS A (entirely fatty nondense breasts) or BI-RADS D (extremely dense). Subjects then underwent multiparametric MRI for analyses of lean tissue fraction, apparent diffusion coefficient and perfusion dynamics. Additionally, investigators used a technique called microdialysis, where a thin catheter was introduced into breast tissue to obtain samples of the microenvironment surrounding cells, with researchers measuring the amounts of different proteins in the fluid.
Results showed that, in comparison with nondense breast tissue, dense breast tissue exhibited a range of different properties linked to tissue microstructure, including increased lean tissue fraction (the amount of tissue in breast that is not fat) slower perfusion dynamics and increased and more heterogenous apparent diffusion coefficient (a measure of the oedema in tissues).
Altogether, the investigators measured levels of 270 proteins and found that levels of 124 of them were elevated in dense breast tissue (P<0.035). These 124 proteins were divided into three groups: one group was mainly involved in the regulation of inflammation (65 proteins); one group related to growth factors/ metabolism (34 proteins); and one group was involved in angiogenesis (25 proteins).
- Regarding the 65 inflammatory proteins, 62 correlated with lean tissue fraction, 59 proteins with apparent diffusion coefficient, and 56 proteins with perfusion tau (a measure of the blood flow in the tissue obtained by analysing the flow of the contrast).
- Regarding the 34 growth factors/ metabolic proteins, 31 proteins correlated with lean tissue fraction, 33 with apparent diffusion coefficient and 29 with perfusion tau.
- Regarding the 25 angiogenic proteins, 18 correlated with lean tissue fraction, 22 with apparent diffusion coefficient and 17 with perfusion tau.
The team have hypothesised that dense breasts may possess disadvantageous microenvironments that promote the transition of anomalous breast cells to cancer, which may provide the explanation for the higher risk of cancer for women with dense breasts. Such an explanation, they suggest, would account for why approximately one in three women in the age group 40 to 50 has precursors to cancer in the breast, yet fewer than 1% in this age group go on to develop cancer.
“The results raise many questions about whether it is possible to reduce the levels of these proteins and reduce the risk of developing cancer. We open possibilities that we have not previously had,” says Dabrosin.
The team now plan a clinical study in women with dense breasts to explore whether the microenvironment can be changed with therapeutics targeting inflammatory proteins, such as non-steroidal anti-inflammatory drugs, as well as therapies aimed at inhibiting growth factor/ metabolic pathways. In these studies, adds Dabrosin, measurements such as apparent diffusion coefficient, lean tissue fraction and perfusion tau could be used to assess whether different drugs reduce the density of breast tissue, and as such might be effective in preventing cancer.
In normal breast development, after menopause breast density decreases and eventually disappears. “We believe that women who maintain breast density after menopause are the group who would most benefit from prevention strategies,” Dabrosin told Cancerworld.
Many questions remain to be answered. “We don’t know what’s the chicken or the egg. Does an inflammatory microenvironment result in increased production of collagen that in turn leads to increased tissue pressure which will further affect the microenvironment? Or is the stroma completely different in individuals with dense breast tissue from the beginning?” asks Dabrosin.