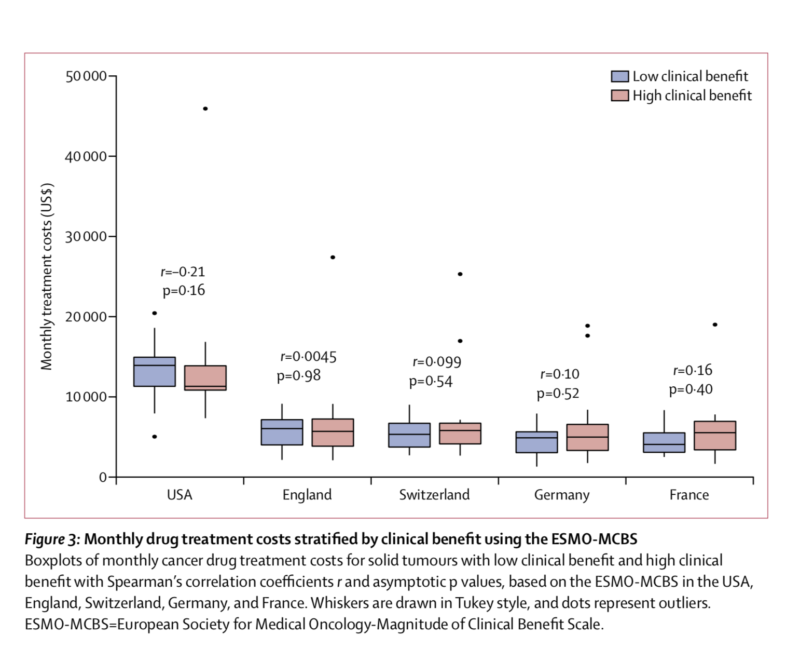
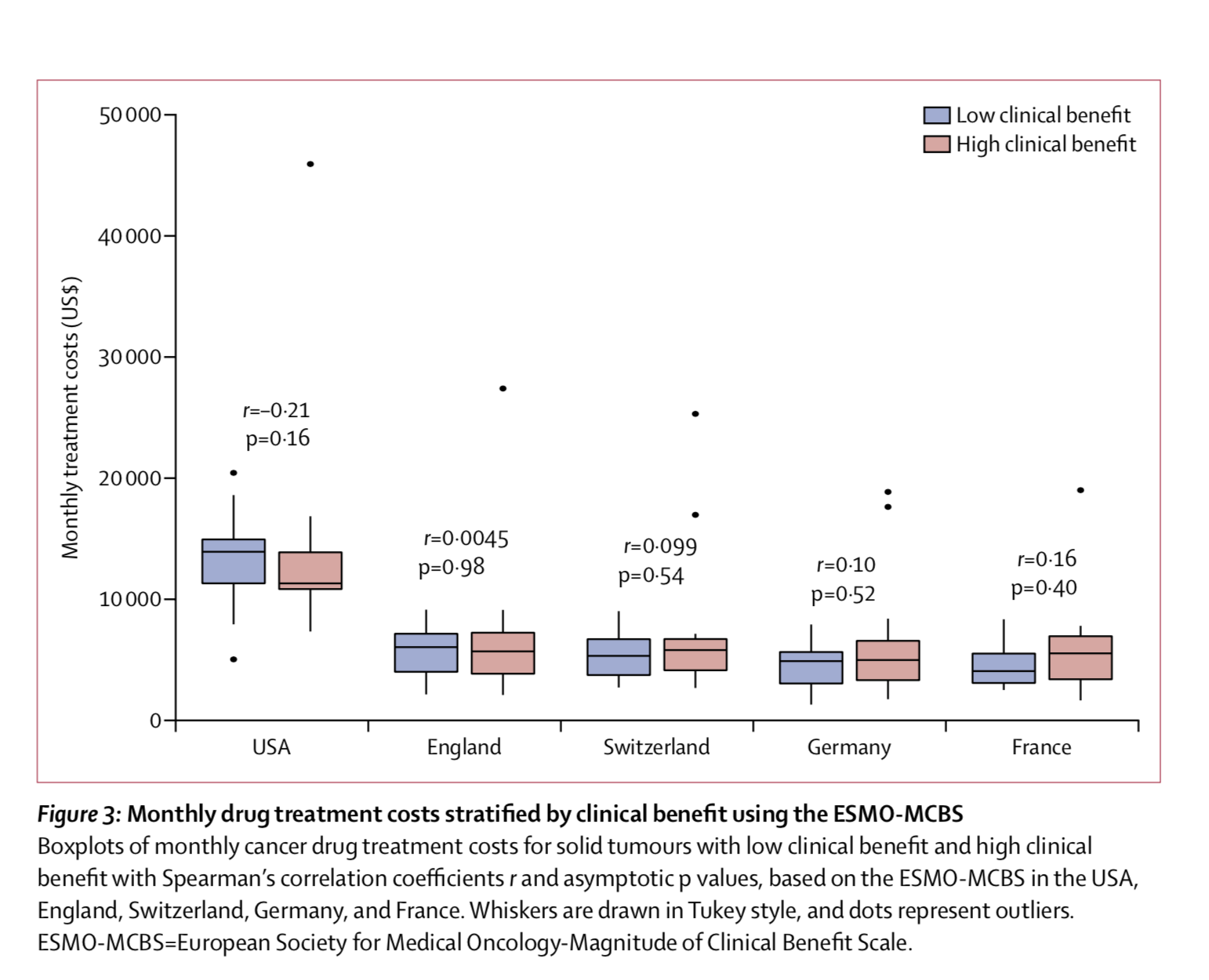
According to an international study just published in Lancet Oncology, the cost of new cancer drugs is not associated to the clinical value of the therapy: “In the USA and European countries, prices of cancer drugs should be better aligned with their clinical importance, such as by prioritising drugs with low or uncertain benefit for price negotiations, to improve access to beneficial drugs and enable finite resources to be used for treatments that offer patients improved outcomes and optimal value” write Kerstin Noëlle Vokinger, professor for health law at the University of Zurich, and colleagues.
Vokinger’s group focused on 65 drugs approved in the last decade for the treatment of solid tumours or haematological malignancies. Monthly treatment costs for each drug were calculated separately for the USA and for four European countries (England, France, Germany, Switzerland). Cancer drug costs were more than double in the USA compared to Europe. This difference is mainly due to the fact that, by contrast with the USA, European countries have policies that allow national authorities to negotiate drug prices with manufacturers.
Strikingly, no significant associations between monthly drug treatment costs and clinical benefit was observed: for a given cancer type, a drug with lower clinical benefit score may have similar or even higher monthly costs compared to another drug with a higher score.
The clinical value of cancer therapies was evaluated using two frameworks developed by the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO). The two tools have moderate concordance, although they were originally developed for different purposes: the ASCO Value Framework (ASCO-VF) was designed to help shared decision making on the most beneficial drug for an individual patient; the ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) was designed to identify cancer therapies that should be made rapidly available in every EU country. “In both the USA and in European countries, pricing for cancer drugs is complicated by the fact that many newly approved cancer drugs have weak or uncertain evidence about their clinical value, often because they are approved on the basis of surrogate endpoints” the author explain. Value frameworks can help identify therapies providing high clinical benefit and are useful tools for policy makers determined to to promote the affordability of new drugs.