The European Cancer Organisation and Organisation of European Cancer Institutes have put forward key principles to guide the development of a European Network of Comprehensive Cancer Centres.
In a position paper published on September 8th, they set out seven recommendations they argue will be key to implementing EU policies on developing and improving Europe’s infrastructure for delivering high-quality cancer care and research.
The recommendations relate specifically to EU policy on developing comprehensive cancer care networks, as spelt out in both the Cancer Mission and in Europe’s Beating Cancer Plan.
The recommendations stress the importance of clearly defined aims. They suggest that that these should cover three areas: reducing inequalities in diagnosis, treatment and care, and access to clinical trials; strengthening quality of translational, clinical and outcomes research; and integrating clinical care and research and evaluating the quality of cancer care throughout.
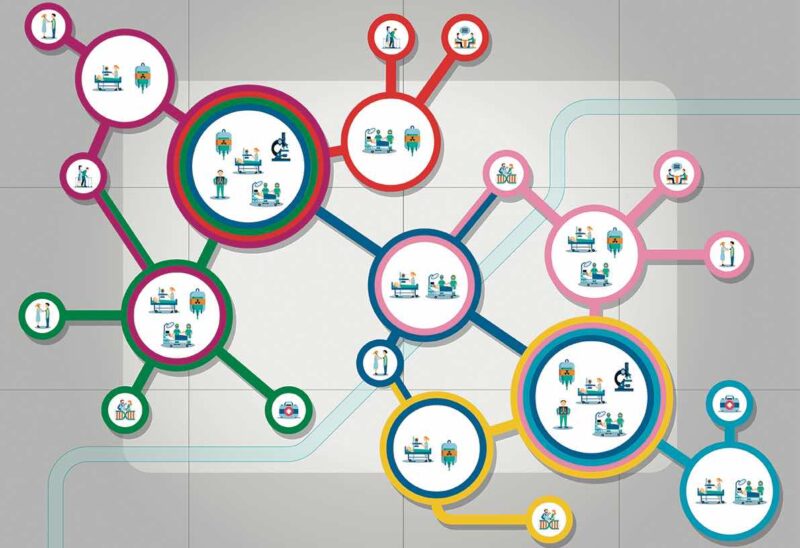
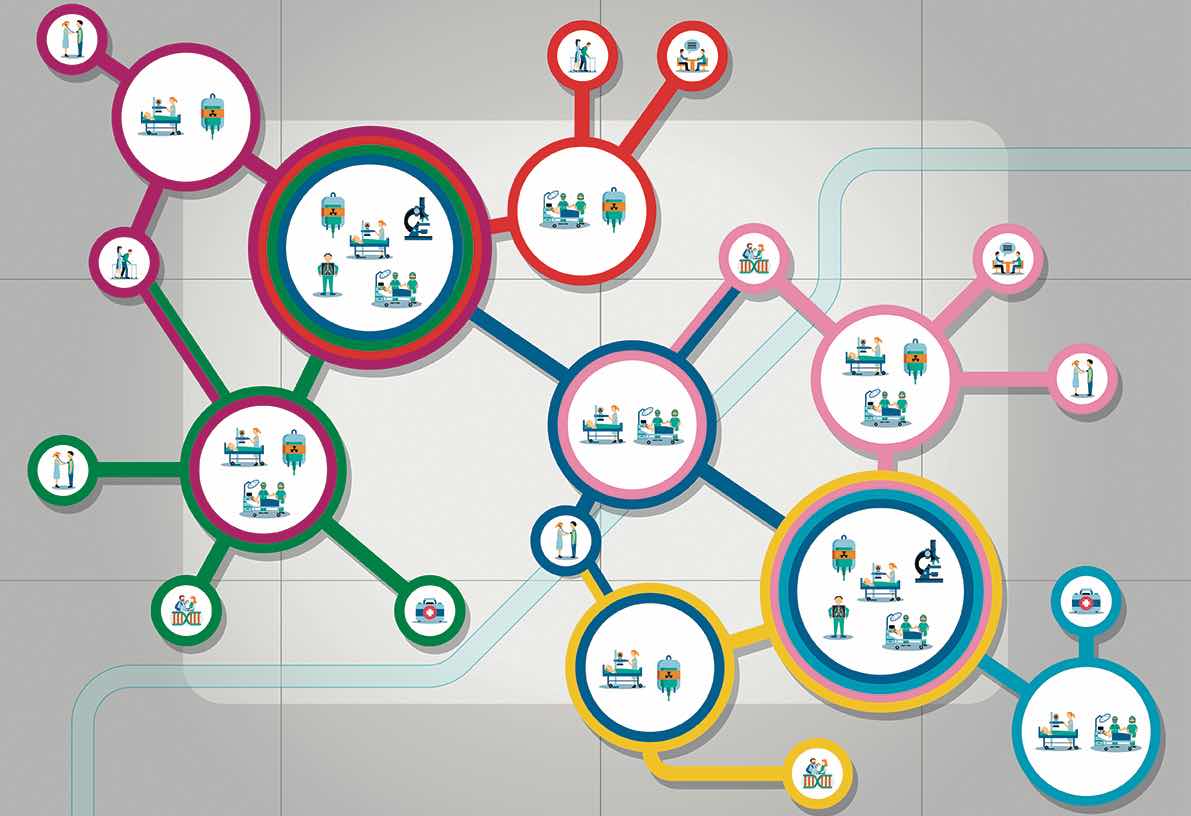
They also stress the importance of clearly defined and sustainable sources of funding to support the development of national Comprehensive Cancer Care Networks. Development plans should start by mapping existing Comprehensive Cancer Centres and needs, they stress, and there should be agreement on the structures and services to be included in such Networks. Their recommendation is for all general hospitals where cancer patients are treated to be formally included, with primary care providers to be “encouraged and supported” to be connected to the Networks.
The position paper, Comprehensive Cancer Care Across the EU: Advancing the Vision, was developed through discussions involving 21 healthcare professional organisations, nine patient organisations and other organisations involved in the European Cancer Organisation’s Quality Cancer Care Network.
One consistent theme was the need to build on effective structures and processes that are already in place across Europe and working well. In terms of developing networked research capacity, for instance, the paper highlights Europe’s strong cancer clinical/translational research infrastructure, and recommends this should be leveraged, with research programmes focused around specific clinical, translation and outcomes questions.
The position paper also points to “existing quality programmes of care and research, and accreditation programmes (at both cancer centre and organ-based levels)”, and recommends these should be affirmed and facilitated, rather than the EU duplicating them, or re-inventing new processes.
The seven recommendations are intended to contribute to discussions at forthcoming Autumn meetings of the Commission, Member States and Parliament on implementation of EU cancer policy.
In presenting the recommendations from the European Cancer Organisation (E.C.O.) and the Organisation of European Cancer Institutes (OECI), Matti Aapro, E.C.O. President, praised the Europe’s Beating Cancer Plan for the attention it pays to “the fundamental infrastructure that underpins quality cancer care,” and for recognising the successful model of comprehensive cancer centres as an approach to be more widely adopted. “We are all excited to have the chance to work with the Commission, Member States and others to make sure this will be the case,” he said.
OECI President Thierry Philip, said his organisation welcomed the opportunity to work with such a broad group of healthcare professional and patient organisations to develop the position paper: “When it comes to the quality of cancer care we are all partners,” he said. “As the EU embarks on the exciting project of constructing an EU Network of Comprehensive Cancer Centres,” he added, “it is important that the cancer community defines their purpose and objectives clearly, so that Europe makes best use of our comprehensive skills and resources to prevent, diagnose, treat, and cure cancer. We hope our policy paper assists in this respect”.