Liver biopsies undertaken at the time of surgery for pancreatic cancer can be used to identify cellular and molecular markers that predict whether early pancreatic cancer will later metastasise to the liver. The study, published in Nature Medicine, June 28, showed that a machine-learning model, trained on information obtained from the biopsies, can predict metastatic outcomes with 78% accuracy.
“If we can predict the timing and location of metastases, that could be a paradigm shift in treating pancreatic cancer, particularly for patients at high risk of metastasis,” study co-senior author David Lyden, tells Cancerworld. “Our paper shows that ‘pre-metastatic niches’ exist at distant organ sites, such as the liver, in patients and opens up an entirely new way to treat metastasising cancers.”
Fewer than one in four cases of pancreatic cancer are amenable to potentially curative resection and, of these, only one in five surgical patients will survive five years. “Thus, reliable strategies for identification of patients at high risk of distant recurrence in the pre-operative setting are urgently needed,” write the authors.
It was Lyden, from Weill Cornell Medical College in New York, who in a paper published in Nature in 2005, first defined the concept of the ‘pre-metastatic niche’, where tumour-secreted factors induce the formation of microenvironments in distant organs conducive to tumour-cell survival. In a second study, published in Nature in 2015, Lyden and his team showed that extracellular vesicles – small membrane-bound vesicles measuring 30 to 100 nm in diameter, with cargoes of proteins, lipids, and nucleic acids – are transported directly from pancreatic cancer tumours to the liver, where they are taken up by Kupffer cells (innate immune cells) and promote pro-inflammatory supporting micro-environments.
The team also showed that, on their surface, extracellular vesicles targeting different organs display different integrins (cell-adhesion proteins), and that the integrin profile acts like a ‘zip code’ directing vesicles to different organ sites. The finding helps explain the mystery of why cancers from one organ metastasise to specific organs (principally liver, lung, brain, and bone). “Our work suggested that the primary tumour cells are able to modify future distant metastatic organ sites, shaping the ‘soil’, described by Steven Paget in his 1889 ‘seed and soil hypothesis’, making it conducive to metastasis,” explains Lyden.
For the current study, Lyden and colleagues obtained liver biopsies from 49 individuals undergoing surgical treatment for localised stage I-III pancreatic cancer (with no signs of metastatic disease at the time). They also collected liver biopsies from 19 people who underwent similar operations for conditions unrelated to cancer, such as removal of benign pancreatic cysts.
Using techniques such as mRNA sequencing, multiplex mass cytometry imaging, and single cell analysis, the team investigated the cellular makeup of the livers of all subjects as well as the ‘spatial geography’ of the immune cells, to determine whether they were clustered together or dispersed throughout the liver.
Patients were followed prospectively (3–8 years post diagnosis) and classified into four different recurrence groups – early metastases (<6 months after resection) or late metastases (>6 months after resection), liver metastases, extrahepatic metastases (metastases outside the liver, largely involving the lung) and disease-free survivors (with no evidence of metastatic disease).
Results showed the following differences between the groups:
- Patients with early metastases (occurring <6 months later) showed abundant neutrophil extracellular traps (NETs), upregulation of the gene encoding sortilin 1, and lack of T cells and natural killer cells.
- Patients with later metastases (occurring >6 months later) had fewer NETs than the first group and they did have T cells and natural killer cells (although they were exhausted, defined by missing key cytokines).
- Patients who had extrahepatic metastasis (mainly lung) had upregulated interferon genes, and plenty of T cells and natural killer cells present.
- Disease free survivors had liver profiles resembling the livers of subjects who did not have cancer, with no NETs and no rise in T cells and natural killer cells.
The investigators then fed information from individual biopsies and patient outcomes into a machine-learning model to train the model to predict outcomes. Taking the data of the individual subjects (disregarding outcomes), they then used the model to predict their individual metastatic outcomes. Results showed that the accuracy for predicting metastatic outcomes was 78% overall, 90% for those who developed early metastasis and 78% for those who did not develop metastasis.
Liver biopsy markers that predict metastasis from pancreatic cancer
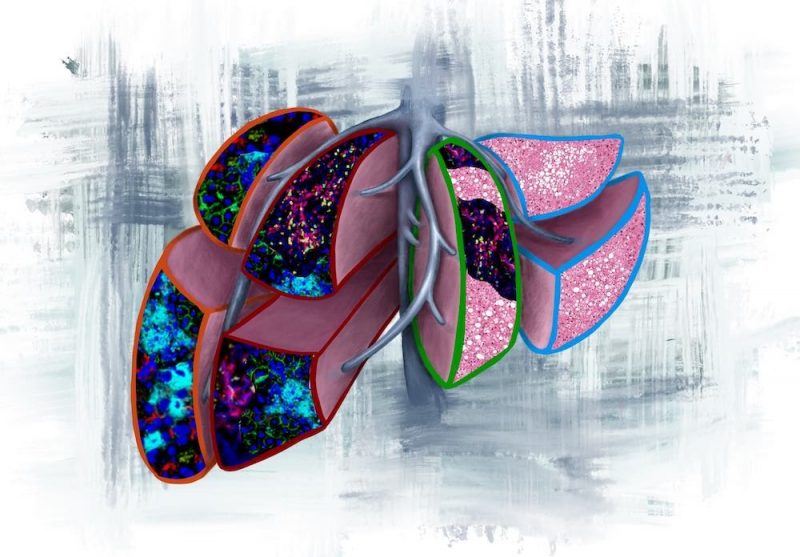
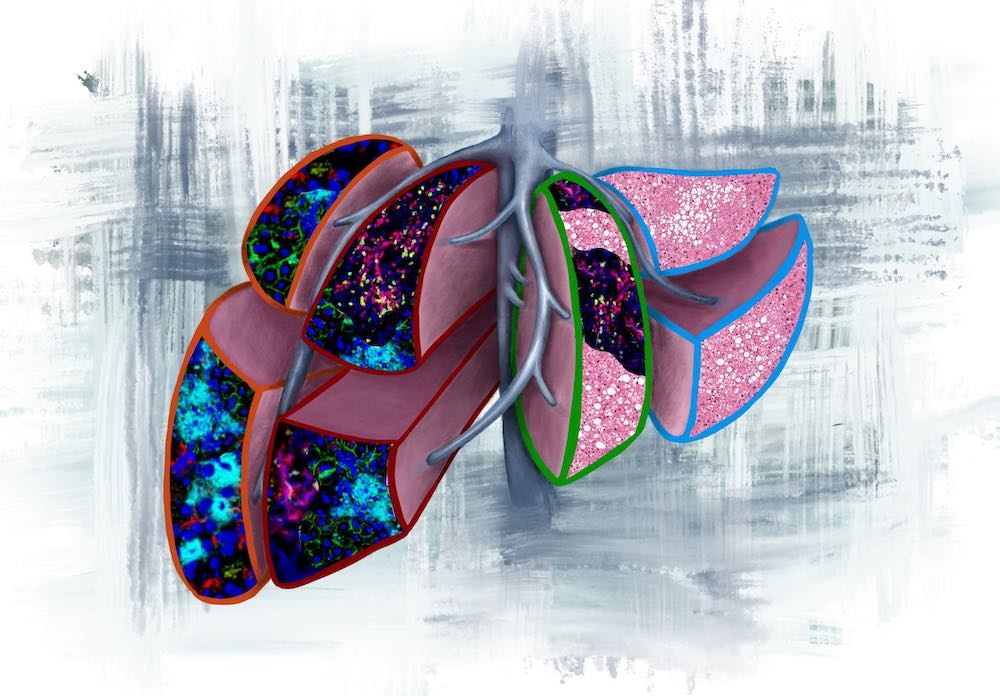
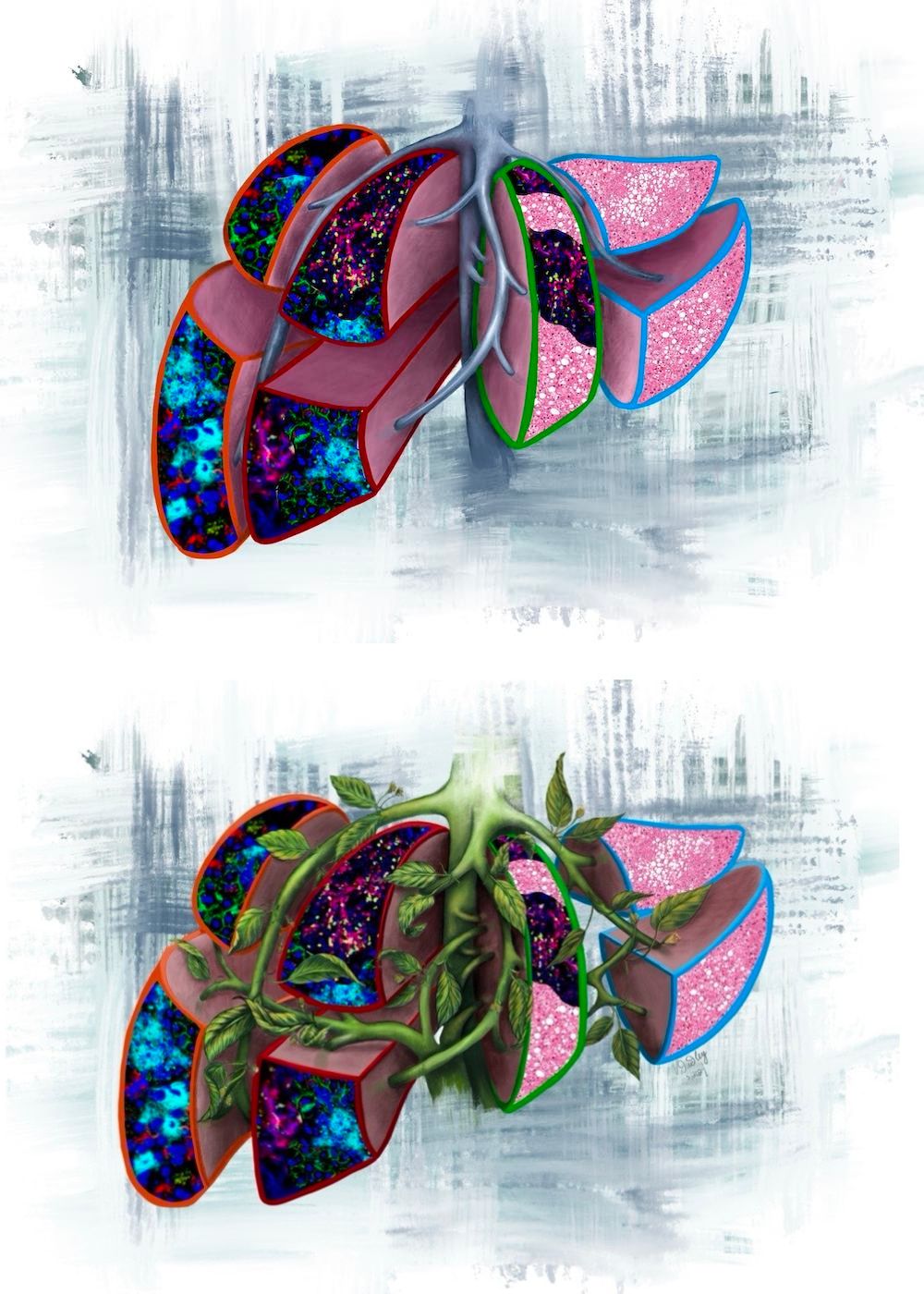 This image illustrates the four prognostic liver lobes in the pre-metastatic liver, as outlined by the colour codes from the current study:
This image illustrates the four prognostic liver lobes in the pre-metastatic liver, as outlined by the colour codes from the current study:
Orange outline: neutrophil extracellular traps (NETs), predicts for liver metastasis <6months.
Red outline: NETs and T cells, predicts for liver metastasis >6months.
Green outline: T cells and fatty liver, predicts for extrahepatic metastasis.
Light blue outline: normal liver with fatty liver, predicts for no evidence of disease.
The version with vines represents tumour-secreted factors (extracellular vesicles) that overwhelm the liver and transform it into the different pathologies.
Image credit: Vanessa Dudley
“Introducing our approach into clinical practice would allow the identification of different subgroups of pancreatic cancer patients whose treatment could be tailored according to their predicted metastatic group and improve the survival of patients on a big scale,” says Linda Bojmar, the first author of the paper, who has since opened her own laboratory in Linköping University, Sweden.
For example, she says, those with predicted late liver metastasis could be offered surgery, and those with predicted extra hepatic metastasis showing a good immune response (plenty of T cells and natural killer cells) could be offered immunotherapy.
The biggest gains, however, are likely to be for those with early liver metastasis, who would probably not derive benefit from surgery but could potentially benefit from neoadjuvant systemic treatments. New therapies are currently in development to specifically target sortilin-1 and NETs (DNAase 1 and PAD4 inhibitors). “NETs are in a constant state of turnover, which means if we could take them away, we might not only prevent the metastasis from forming, but also attenuate metastases already there,” says Lyden.
Next the team hope to validate the machine-learning model in a large independent cohort of patients with pancreatic cancer, and to then use the model to identify a smaller number of prognostic markers to make the approach more accessible for clinical practice.
With the aim of better understanding the biogenesis of the extracellular vesicles, they also plan to isolate extracellular vesicles from the four different groups to determine whether there are differences.
The biopsy approach, Lyden believes, could be extended to other potential sites of metastasis. This could include the lung and bone, but probably not the brain. “Instead, it would be more practical to develop radiographic imaging tools to identify pre-metastatic niches in the brain, or better still blood biopsies that could avoid the need for surgery,” he says.