“It’s extraordinary that screening for the biggest cancer killer is not available in most of Europe,” says Anne Marie Baird, President of Lung Cancer Europe (LuCE). “Lung cancer causes more deaths in Europe than breast, colorectal and cervical cancers combined, yet for these cancers, population based screening programmes are readily available.” Delays in implementing screening for lung cancer have resulted in the loss of many lives, she adds, “Mortality from lung cancer is huge in Europe, accounting for 28% of all cancer deaths. In 2018, 470,000 people in the EU were diagnosed with lung cancer and around 338,000 died from it… These aren’t just statistics, each patient who dies represents an individual loss to their families and friends.”
Lung cancer is a particularly fatal malignancy because it is so hard to detect in its early stages. Almost three quarters of lung cancer patients present with late-stage disease where treatments have little effect on mortality. While more than seven in ten people diagnosed with early-stage lung cancer are still alive five years on, for those diagnosed at a late stage that figure is fewer than one in ten. That explains the importance of an effective screening programme, says John Field, Director of Research at the Roy Castle Lung Cancer Programme at the University of Liverpool, UK. “The overriding objective of lung cancer screening is to shift timing of diagnosis to an earlier point so that disease is localised to the lung allowing surgery or stereotactic radiotherapy to eradicate the cancer.”
“NELSON put paid to objections that lung screening had not shown benefits in a large European trial”
With publication of the results of the NELSON trial in the New England Journal of Medicine, January 2020, evidence for the benefit of lung cancer screening with low dose computed tomography (LDCT) is now considered indisputable. “NELSON put paid to objections that lung screening had not shown benefits in a large European trial,” explains Field.
In this Dutch‒Belgian trial, nearly 16,000 individuals considered at high risk for lung cancer underwent either four rounds of LDCT or no screening. Results at 10 years show participants in the screening group had a 26% reduction in death from lung cancer compared with those in the control group.
These positive results support the findings of the 2011 US-based National Lung Cancer Screening Trial (NLST), which show a 20% reduction in cancer mortality for those screened with three rounds of annual LDCT screening versus chest X-ray. The NLST study is now considered to have underestimated benefits, as all participants had a scan of some kind (either X-ray or LDCT). Other smaller European-based studies demonstrating survival benefits for lung cancer screening include the Multicentre Italian Lung Detection (MILD) trial, the German Lung Cancer Screening Intervention Trial (LUSI), and the UK Lung Cancer Screening Trial (UKLS).
In 2013, as a direct result of the US NLST trial, the US Preventative Services Task Force (USPSTF) recommended current or former smokers meeting defined criteria should be offered annual screening. The latest US guidance, which was updated this year (2021) and is followed by health insurance groups and Medicare and Medicaid, is that people aged 50 or over with a smoking history of 20 pack years (i.e. who have smoked one pack a day for 20 years) should be offered annual LDCT scans.
Situation across Europe
Europe has proved altogether more wary of lung cancer screening, with countries adopting different attitudes ranging from genuine interest to downright hostility. In October 2020 Croatia became the first country in European to launch a national lung cancer screening programme for all eligible citizens. In contrast, countries like the UK, Poland and France have chosen instead to start by setting up pilot schemes at a few centres first, with a view to tweaking the service as they go along, and performing analyses demonstrating cost-effectiveness to convince payers. Other countries, such as Spain, have no pilot initiatives planned, with lung cancer screening available only on a private basis or as part of a European clinical trial.
Referring to Croatia’s decision to offer a national service from the outset, Ante Marušić, a thoracic radiologist from University Hospital Centre, Zagreb, says the small size of the country was a factor. “While we knew it would be impossible to foresee everything from the start, as Croatia has just four million people and 16 screening centres we felt it would be feasible to adapt the service as we went along.”
“We waited 15 years for the results of NELSON and felt it would be unfair to make people wait any longer for widespread screening”
Another key factor in the decision to launch straight into the real thing, adds Marušić, was the importance of providing equal access to all eligible people. “We waited 15 years for the results of NELSON and felt it would be unfair to make people wait any longer for widespread screening.”
With a population of around 68 million, introducing lung cancer screening into the UK represents an altogether more complex undertaking, says Field. “To deliver a full national screening service there are multiple aspects that need to be well integrated with each other. Running pilots allows countries to design screening programmes that are both appropriate and cost-effective for their own particular health systems and evolve the service gradually.”
Witold Rzyman, a thoracic surgeon from the Medical University of Gdansk, Poland, agrees that countries need to run their own pilots and cannot just rely on information obtained from pilots elsewhere. “Each country has different healthcare systems, making it necessary to undertake individual cost-effectiveness studies.”
As Field points out, differences in culture can be as important as differences in health systems, when it comes to designing an effective lung screening service. “For optimum uptakes you need to consider different ways of approaching people to take part in screening, whether they respond best to receiving letters or telephone calls, and the best terminology to get across concepts of risk to that particular population.”
This can make cross-border collaborations challenging. In Europe, the only cross-border initiative is the 4-IN-THE-LUNG RUN (4ITLR) study, funded by the European Horizon 2020 programme, which aims to determine whether screening intervals of two years after a negative baseline scan are as effective as annual screening. Results of 4ITLR, which plans to screen 26,000 people at sites in the Netherlands, Germany, Spain, Italy and France, will have important implications for containing costs, thereby making screening more attractive to payers.
There are fundamental differences between trials and pilots, explains Michael Davies, who works with Field at the University of Liverpool. “Trials are designed to answer specific questions, whereas pilots are used to demonstrate whether you can achieve good results in the real world when you upscale screening to provide a service.”
The wide-ranging topics that need to be addressed include: Who should be eligible for screening? What should the screening intervals be? How to incorporate smoking cessation? How to deal with incidental findings? ‒ as well as getting the patient information literature right, and establishing central registries. Other challenges include assembling multidisciplinary teams to discuss suspicious results, which would need to include all relevant specialities, including pulmonologists, thoracic surgeons, radiologists, pathologists and lung cancer nurses. From the radiological perspective, organisation gets even more complicated with the need to standardise diagnostic criteria for screen-detected nodules and implement quality assurance to ensure that CT images are undertaken, recorded and reported to uniform high standards. Training programmes are also needed for radiologists to ensure a suitably trained workforce is in place to deliver the service.
Balancing benefits against risks
The scale of infrastructure investment involved requires considerable commitment, which may prove a step too far for some European countries, which historically have a mistrust of screening. Screening, it should never be forgotten, carries risks as well as benefits. Valid concerns include: exposing healthy people to radiation (although the advent of LDCT reduced radiation doses 10 fold); the high incidence of false positives; the anxiety and stress that can accompany screening; and the risk that people can be exposed to unnecessary procedures such as biopsy, bronchoscopy and surgery. “Caution is needed because lung cancer biopsies are invasive and expose people to complications of pneumothorax. This poses a completely different level of risk from say a breast biopsy,” says Helmut Prosch, a thoracic radiologist from University of Vienna, Austria. Even when malignancy is accurately detected, there are concerns that, left to its own devices, the cancer might not have gone on to cause symptoms and limit life expectancy.
A number of research developments are taking place to boost the likelihood of benefit and lower risk associated with lung cancer screening.
Targeted screening
One approach to limiting risk is to focus screening on individuals at greatest risk of developing cancer. While the NELSON and NLST studies used criteria based on the number of years participants had smoked (known as pack years), the UKLS study incorporated the Liverpool Lung Project (LLP) risk prediction model. The model had been generated by Field and colleagues, who compared data from 579 lung cancer cases and 1,157 age- and sex-matched controls. From a Cox proportional hazards model, the team found lung cancer risk factors included age, sex, smoking duration, history of pneumonia, non-lung cancer, asbestos exposure and family history of lung cancer. “Incorporating the model into screening offers the advantage of minimising unnecessary interventions from those at lower risk and providing a more cost-effective programme,” explains Field.
Another risk model used is the Prostate, Lung Colorectal, and Ovarian (PLCO) programme, which has a slightly different emphasis, including socio-economic status and body mass index. The NHS England Targeted Lung Health Check Programme is incorporating both the LLP and PLCO risk models in their pilot, to explore which is the most effective. “Whatever model is used, it needs to be remembered that risks for developing lung cancer vary over time, due to changes in risk factors, like age. Consequently people who have been deemed ineligible may become eligible, underlying the importance of regular risk assessments,” says Field.
Engaging the at risk population
For screening services to be effective they need to engage target populations. The fundamental importance of understanding your target audience is clear from the salutary experience of the uptake of lung screening in the US. “Despite having national screening programmes endorsed by their medical societies, only around 5% of high-risk Americans took up the opportunity of lung screening,” says Field.
One major obstacle that needs to be overcome is deep-rooted feelings of shame around smoking. “Smokers assimilate the stigma of lung cancer and are afraid to come forward for fear of being judged,” says Luis Seijo, a pulmonologist from the Universidad de Navarra, Madrid. “In asymptomatic healthy people, there’s also a strong instinct to avoid screening due to fears of receiving a life-threatening diagnosis,” he adds.
“Dissociating screening from the word ‘cancer’ really helps to frame screening in a more positive light”
Simply changing the name to the more neutral term ‘Lung Health Check’ was sufficient to diminish hesitancy, investigators in the UKLS study discovered. “Dissociating screening from the word ‘cancer’ really helps to frame screening in a more positive light,” Seijo agrees.
Lung Health Checks become as good as their name if the appointment also screens for other tobacco-related diseases, such as chronic obstructive pulmonary disease (COPD) and coronary artery disease. These inclusions again deliver additional benefits of making screening more cost-effective for health services.
Another major challenge is how to reach people in more deprived areas, which is where lung cancer is most prevalent says the thoracic radiologist from Vienna, Prosch. “The problem we perpetually face is that it’s the most affluent members of society who put themselves forward for screening, yet these are the people with least risk of lung cancer.”
Phil Crosbie, a respiratory medicine consultant from Wythenshawe Hospital, Manchester, argues that the experience of Manchester’s ‘Lung Health Check’ pilot shows it is perfectly feasible to reach deprived populations so long as the service is designed appropriately. “Instead of waiting for people to come to us in hospital clinics we designed our service to be as convenient as possible, by taking our LDCT scanners out into the community,” explains Crosbie. “We offered one-stop lung health checks in vans in supermarket car parks, located near to where people live with the added advantage of providing plenty of parking.”
For the initiative, people aged between 55 and 74 years from 14 GP practices in deprived areas were sent letters inviting them for screening if they smoked. The assessment, conducted by lung specialist nurses, involved a discussion about symptoms, a breathing test (spirometry), and calculation of individual cancer risk. Anyone found to be at high risk of lung cancer was then invited to have an immediate LDCT scan in a mobile scanner.
“We offered one-stop lung health checks in vans in supermarket car parks, near where people live and with plenty of parking”
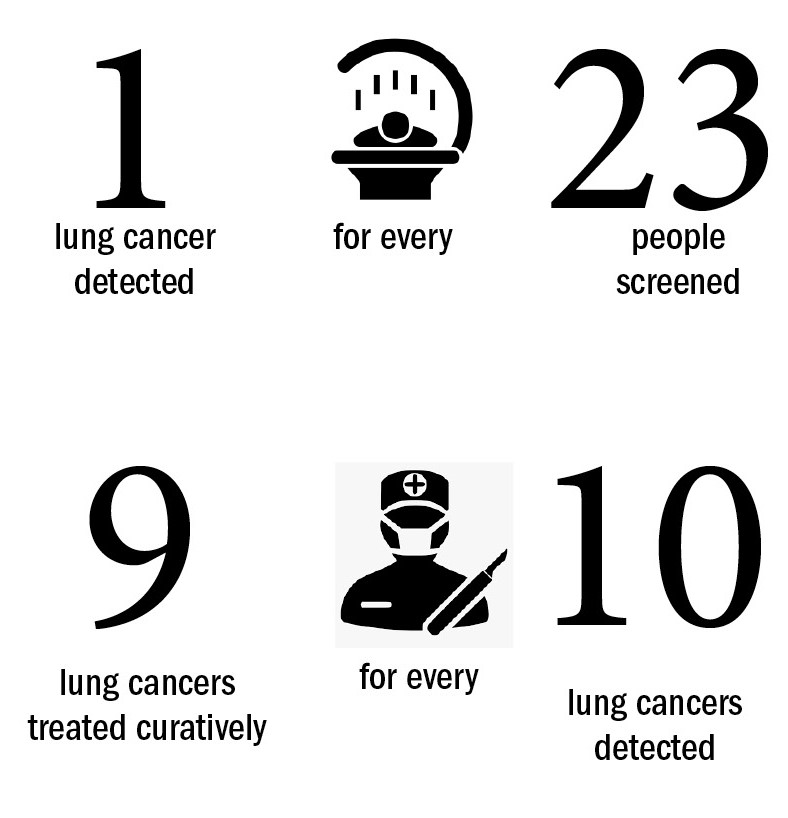
Results show that one lung cancer was detected for every 23 people who underwent two rounds of LDCT scans. “This level was higher than any of the large international trials, showing additional benefits can be achieved by targeting deprived populations,” says Crosbie.
The study also showed almost eight out of ten cancers picked up this way were stage 1 (79%), and almost nine out of ten people diagnosed with lung cancer (89%) were offered curative treatment. The Manchester data proved so inspirational that NHS England announced that they will be operating scanning trucks from supermarket car parks in all their 20 screening pilots.
Aside from the compassionate aspect of saving more people, increasing the yield of lung cancer identification undoubtedly shifts the cost-effectiveness dial positively in the right direction, making screening a more attractive economical prospect for health care providers.
Manchester Lung Health Check pilot
A teachable moment
Another innovation with potential to both save more lives and make initiatives more cost effective is applying smoking cessation in tandem with screening. Screening offers a ‘teachable moment’, providing an opportunity to motivate individuals to adopt risk-reducing health behaviours. “Screening programmes need to start supporting smokers properly to quit, not blaming them for their habit,” says Marie-Pierre Revel, a radiologist from Cochin University Hospital, Paris. “We need to research incorporating smoking cessation properly and realise that smoking is an extreme addiction that smokers don’t do on a whim but because they have no choice.”
“Screening programmes need to start supporting smokers properly to quit, not blaming them for their habit”
Results around smoking cessation have proved mixed. Data from the UKLS trial shows enrolment in lung cancer screening programmes had an overall positive effect on smoking cessation, especially for those requiring additional clinical investigations. However, neither the NELSON study nor Danish Lung Cancer Screening trial found differences in smoking cessation between screening and control groups. Receiving a normal scan result, it has been suggested, may induce a false sense of security in participants who feel invincible against the harmful effects of smoking.
“But none of these studies had on-site smoking cessation interventions bundled together with screening,” says Matthew Callister, a respiratory medicine consultant from Leeds Teaching Hospital, UK. “On the whole, participants were just given cards directing them to local smoking cessation services. In reality it’s all too easy for people to lose the card or become too busy with other things, so the proportion making contact is likely to be small.”
Participants who are still smoking are offered access to smoking cessation counsellors on the screening van immediately prior to undergoing scans
To increase uptake, the Yorkshire Enhanced Stop Smoking (YESS) study, running in Leeds, is evaluating adding a personalised smoking cessation intervention to lung cancer screening. In this study, funded by Yorkshire Cancer Research, participants who are still smoking are offered access to smoking cessation counsellors on the screening van immediately prior to undergoing scans. “It’s opt out, with the smoking cessation room just metres from the scanner, making it easy for people to engage with the service,” explains Callister, adding vans have been specially configured to make space for private smoking cessation rooms.
Lung Health Check patient management system
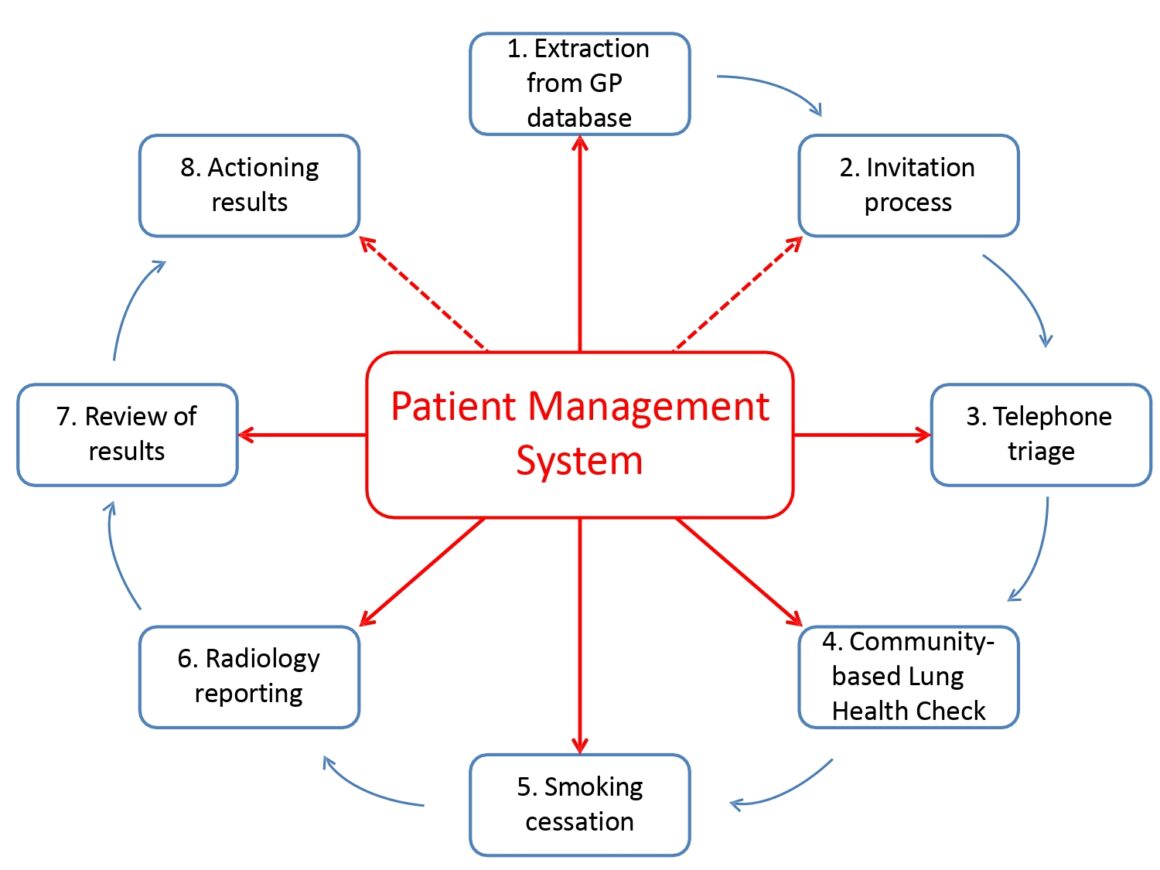
The smoking cessation programme, provided for four weeks, includes behavioural support over the telephone, nicotine replacement therapy, pharmacotherapy, and /or commercially available e-cigarettes. Then, after four weeks, participants who are happy to take part are randomised to either continue best practice of ongoing support for a further eight weeks or get best practice plus an individualised report on their heart and lung damage that has occurred as a direct result of smoking. “We give them a pamphlet with images from CT scans of their own heart highlighting areas of coronary calcification, and their lungs highlighting areas of damage resulting from emphysema,” says Callister (see fig.2, R L Murray et al BMJ Open, 2020). “The concept is a strong visual message – if you stop smoking you’ll prevent other areas of your lung from being similarly damaged.”
The case for a concerted European approach
The absence of any mention of lung cancer screening from Europe’s Beating Cancer Plan is seen by many as a missed opportunity. While the plan, published February 2021, recommends that EU member states ensure that nine in ten of those eligible for breast, cervical and colon screening receive it by 2025, no mention is made of lung cancer. The Covid-19 pandemic has also set back efforts to progress the cancer screening agenda; not everyone is happy with the line taken by organisations such as the European Society for Medical Oncology, which issued a consensus statement indicating enrolment into screening programmes could be deferred to optimise allocation of overstretched resources.
Across Europe, countries with plans for pilots (such as the UK, France and Poland) have been forced to delay their initiatives. In the US, where lung screening has been widely available for a number of years, a recent study showed annual LDCT screening had dropped to almost one quarter of the levels seen in pre-Covid times.
The need for a concerted European approach to lung cancer screening was identified at the European Alliance for Personalised Medicine (EAPM) roundtable meeting, held in December 2020. Delegates suggested lack of European-level involvement perpetuated national divergences of approach. “We felt having the European parliament publish a statement supporting lung cancer screening would deliver the biggest boost to screening,” says Seijo, who participated in the roundtable. Such a statement, he adds, would put pressure on countries like Spain to overcome their current inertia around screening. Field agrees, “Even better,” he says, “would be for the European Union to provide funding for member states to set up their own pilots.”
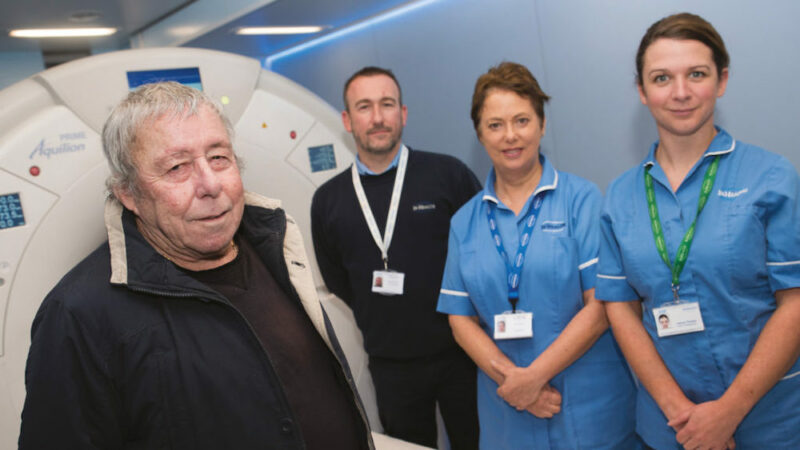
Featured image: NHS England Lung Health Check pilot schemes offer low-dose CT scans to people deemed to be at high risk of lung cancer. Pictured here is Bill Simpson, who was diagnosed with early stage lung cancer in October 2017 via a screening pilot in Strelley, Nottingham, and was successfully treated. With him are members of the Citycare respiratory team, Joanne Adkin and Emma Waring, together with Safiy Karim, Clinical Commissioning Group (CCG) Cancer Lead for Nottingham City
Picture credit: Mansfield and Ashfield Chad