There was a time when all that oncologists treating solid tumours needed to know about leukocytes was how to measure the damage that cytotoxic drugs inflicted on their patients’ white blood cell count and their capacity to fight off infections.
With the introduction of monoclonal antibodies, and more recent molecular discoveries, that is all changing. As immunotherapies expand their role to an ever-widening range of cancer indications, oncologists are quickly becoming familiar with the concepts and the language: cytotoxic T cells, CAR T cells, immune checkpoint blockade, are now part of everyday oncology vocabulary.
A new player is now set to find a place within the cancer treatment armamentarium. Natural killer cells (NKs) have a distinct role within our astonishingly complex immune system. Trials are underway to explore the benefits of mobilising them alongside T cells, to seek out and kill elusive cancer cells.
Our body’s many lines of defence
Our immune systems can deploy a range of tactical assets to defend our health in the face of constant attack by external enemies and by rogue elements within our own bodies. The first line of defence of the organism is mainly physical: skin, mucous barriers, the gastric acid. Once the barricades are breached, other defensive weaponry is brought into play. These comprise mainly a variety of white blood cells, which share responsibility for recognising the enemy and killing it, without damaging healthy cells. Our second line of defence consists of neutrophil granulocytes and macrophages ‒ innate immunity ‒ which are capable of recognising general ‘enemies’, but do not differentiate between different types of enemy or mount attacks targeted at their specific characteristics.
The eradication of serious infections requires a third line of defence, B and T lymphocytes ‒ our adaptive immunity. These are highly trained combat units, each of which specialises in a specific infection. T and B lymphocytes are accordingly never activated en masse; deployment is limited to the units most suitable for dealing with the pathogen in question. They are selected to never attack a healthy cell. If that command fails, autoimmunity is the consequence. The presence and identity of the specific infection is flagged up by antigen-presenting cells (dendritic, Langerhans, macrophages), which present a segment of viral or defective protein by means of a group of genes known as the major histocompatibility complex (MHC).
These lines of defence work well against many attackers. But there are forms of infection, as with herpes simplex, for instance, where the virus is able to skilfully hide any trace of its presence. It does this by blocking the infected cell from exposing on its surface any protein that could alert the immune system to its infected state. This same deception is practised by cancer cells, which elude immune detection by not presenting their hostile face.
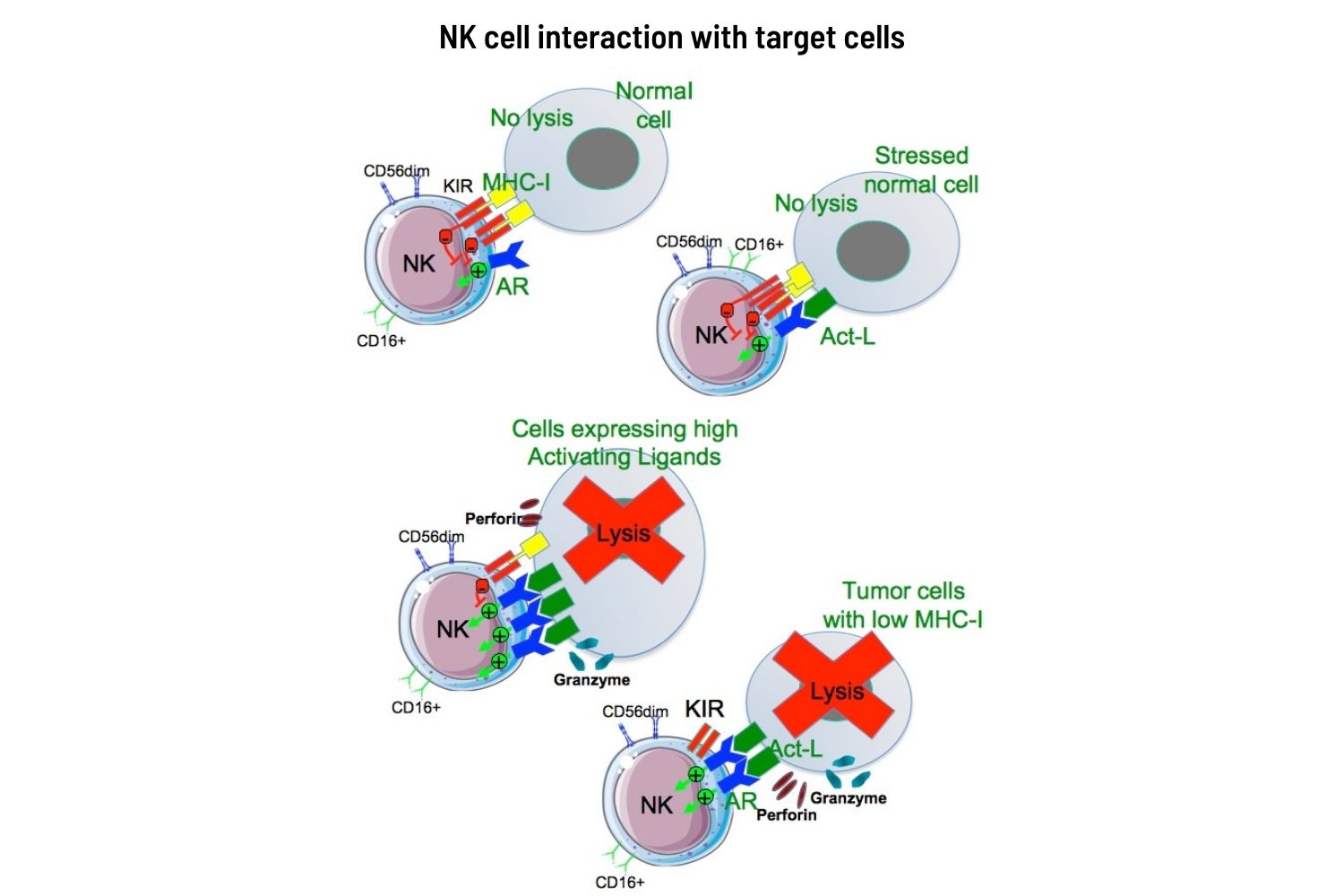
This is where an intermediate line of defence ‒ our NK cells ‒ come into their own. NKs have the unique ability to recognise not the ‘enemy’, but ‘non-friends’ ‒ namely cells that have lost their identity. They are able to specifically kill tumour cells without the priming or prior activation required by T or B cells.
The unique capabilities of NK cells
B and T lymphocytes identify the invaders by recognising proteins that the virus, or the cancer, induces to appear on the cell surface, or through antigen presenting cells, via ‘presentation’ in MHC, NKs, by contrast recognise allies, and suppress ‒ almost indiscriminately ‒ any cells that have lost their identity (dubbed ‘missing self’ by Klas Kärre who identified the phenomenon). The mechanisms that mediate this action are a fascinating and elegant example of how self-control is at the basis of… control.
What the NKs are looking for are molecules of the family of MHC class I. These act as the identity card of a cell and alert the specific immune system (T and B lymphocytes) to intervene if a pathological alteration occurs. When viruses – or tumours – are able to block the production of MHC class I molecules, they succeed in hiding their enemy status from the specific immune system. The NK cells are not so easily fooled. When they see a cell that is not expressing MHC class I family molecules, they identify it as ‘clandestine’ – carrying no ID. And they swing into action.
Constantly patrolling our bodies, these ‘armed police’ check out every passing cell looking for any that are failing to express MHC. They are looking for infected cells or tumours that are carrying no ID.
The receptors in charge of verifying the MHC are called killer inhibitor receptors (KIR). If the right MHC is present, the NK cell moves on. If not, it launches an immediate and lethal attack, using perforin ‒ a perforating enzyme that destroys the membrane of the targeted cell, and granzyme A and B, which destroy the inside of the cell.
This defence system, based on an indirect recognition of danger, plays an essential role in all acute viral infections, because it can kick in immediately, giving T lymphocytes the time they need to fully activate (at least 2‒3 days) and giving B lymphocytes the time they need to produce protective immunoglobulins (around 5 days).
NKs prove indispensable when enemies become too numerous and heterogeneous to be recognised individually. In the chaos of battle ‒ as military commanders know ‒ it can be easier to identify allies and attack the rest.
A new tool in immunotherapy?
Killing off cells that have lost their identity cards is not the only way that NKs protect us. It seems they also carry receptors that are able to pick up signals from infected or cancerous cells that the specific immune system is unable to see (which may further explain why NKs are able to kill only enemy cells and never allies).
The natural cytotoxicity receptors (NCRs) on the surface of NK cells were first identified by Lorenzo and Alessandro Moretta and their groups nearly 20 years ago, in a series of lysis experiments using human NK cells. NCRs are hypothesised to bind to many cellular ligands that are implicated in NK surveillance of tumour cells. Many of these interactions (as well as adhesion molecule roles, discovered by Angela Santoni) have been shown to evoke the cytotoxic and cytokine-secreting functions of NK cells.
These capabilities have long excited interest in the potential for NK cell-based anti-tumour therapies. While the great majority of cell-mediated immunotherapies currently in use focus on T cells, NK cell-based therapies are rapidly emerging in research and in the clinic. NK-based adoptive immunotherapy has already demonstrated some success in haematological cancers including acute leukaemia and myeloid malignancies.
Coming down the line we can expect to see the now-familiar strategy of checkpoint blockade extended to help NK cells positively identify cancer cells and attack them using their perforin and granzyme B cytotoxic weaponry. The PD-1 checkpoint protein, which is the target of certain current immunotherapies, is expressed not only by tumour-associated T cells but also by NK cells. Recent studies have proposed that inhibition of the PD-L1/PD-1 axis could therefore activate NK cells as well, enabling them to play a crucial effector role against MHC class I-deficient tumours, which are undetectable by T cells.
Aside from PD-1 ‒ and KIRs, LIRs and NKG2A, which have long been known about ‒ new research is now revealing multiple inhibitory receptors on NK cells, including, TIGIT, TIM-3, LAG-3, CD96, and IL-1R8, which all present potential targets for checkpoint blockade. Novel engineering strategies are also being developed for targeting solid tumours with NK cells, such as production of chimeric-antigen-receptor-engineered natural killer cells (CAR-NK).
Overcoming challenges
As with all new therapies, however, we may still have a lot to learn before we know how to use them to best effect.
One potential challenge is that, under certain conditions, NK cells may have a tendency to ‘defect’ to the enemy, making them potentially unreliable assets. Instead of exerting an anti-viral and anti-tumour effect, they can change their role to one of support by both losing cytotoxic capability and promoting inflammation and tumour angiogenesis.
In the case of cancer, the circumstances that trigger such defections are closely tied to interactions with the various cellular components of the tumour microenvironment. Cell-to-cell contact, cytokines, chemokines, immunomodulatory molecules, extracellular vesicles, can all play a role in inducing NK cells to ‘polarise’ to exhibiting more pro-tumorigenic properties, leading to promotion of proliferation instead of elimination of tumour cells.
The conversion of NK cells from anti- to pro-tumour ones can be connected to a behaviour that in healthy bodies is associated with pregnancy, where the embryo requires a nurturing environment and also protection from being rejected by the maternal immune system as a quasi ‘foreign body’. My own research group has spent many years investigating a subset of NK cell associated with tumours that has properties similar to the ‘decidual’ NK cells present in the uterus lining during pregnancy, and which differ in important ways from NKs found in the peripheral blood and tissues.
Decidual NK cells accumulate at the foetal-maternal interface and represent 70% of immune cells in the decidua at first trimester pregnancy. They regulate trophoblast invasion ‒ the development of the layer of tissue that supplies the embryo with nourishment and later on forms the major part of the placenta ‒ and they induce vascular remodelling and spiral artery formation, by producing pro-angiogenic cytokines and chemokines (including VEGF and CXCL8).
We and other groups have described a subset of NK cells in cancer patients that have features in common with decidual NK, including expression of CD9, CD49a, and CXCR3, being poorly cytotoxic and pro-angiogenic, and mimicking the decidual nurturing role. The tumour microenvironment seems to have induced ‘good’ NK cells to change to be like decidual NK cells, which consequently start feeding tumour cells instead of lysing them.
One approach to countering the ‘decidualisation’ of NK cells could be to ‘repolarise’ decidualised cells via inhibition of the TGF-β axis, with e.g. galunisertib. This could be expected to remove a major obstacle to the effective use of NK cell immunotherapy against cancer. Other potential targets for repolarising NK cells include NKG2A, glycodelin and galectin-1.
There are excellent reasons to be optimistic about the prospects of harnessing the unique defensive capacities of NK cells as the next generation of immunotherapy. But understanding ‒ and countering ‒ how interactions with elements in the tumour microenvironment might change their behaviour from cancer killer to cancer nurturer, will be important to getting it right. This challenge is now the focus of efforts to ensure that the NK cell-based anti-cancer therapies beginning to emerge in the clinic can be used to maximum effect.
Illustration by: Maddalena Carrai
The AACR journal Cancer Discovery dedicated the first issue of 2021 to NK cells in cancer, including contributions by Eric Vivier ‒ a giant in the field ‒ on tumour-infiltrating NK cells, and by Katayoun Rezvani on the outlook for new CAR-based therapies with a focus on CAR NK cells in the race against cancer, accompanied by our In Focus article on nurturing NK cells.












