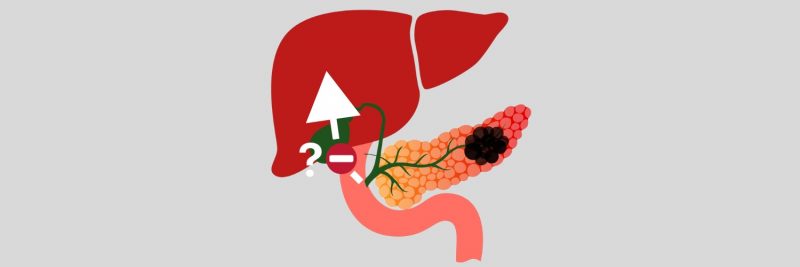
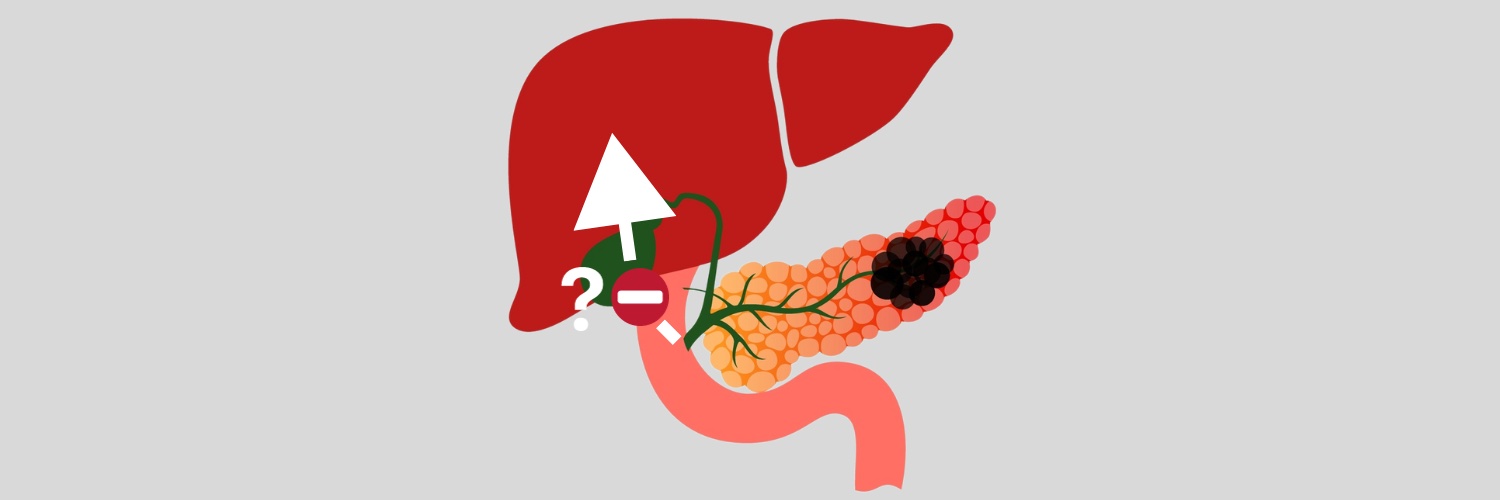
A mechanism that facilitates pancreatic cancer cells spreading to the liver has been elucidated. The study, published in Cell Reports, 2 November, identifies a ‘therapeutic vulnerability’ where the protein Netrin-1, which is upregulated when pancreatic cancer cells metastasise to the liver, can be suppressed with a monoclonal antibody.
“By studying pancreatic cancer that had spread to the liver, and by identifying the molecules and pathways driving that biology, we discovered a novel therapeutic strategy with the potential to treat metastatic disease,” says Darren Carpizo, the first author, from the University of Rochester Medical Center, Rochester, New York. “We showed that Netrin-1 becomes upregulated when pancreatic cancer spreads to the liver, helping metastatic tumour cells to survive, and that if Netrin-1 signalling is inhibited the tumour cells die.”
A defining feature of pancreatic adenocarcinoma (PDAC) is its tendency to undergo early metastatic spread. At the time of diagnosis around 85% of patients will already have stage IV disease, and more than 80% of patients who undergo curative-intent surgery relapse due to the burden of tumour cells present at the time of surgery. An additional issue is that, once pancreatic cancer cells have reached the liver, they undergo epigenetic changes allowing them to survive as disseminated tumour cells in environments that would otherwise be inhospitable.
Recent studies have suggested that metastatic pancreatic cancer is driven by global epigenetic reprogramming that confers survival advantages, rather than by metastasis-specific gene mutations. Netrin-1 is a secreted, laminin-like protein that was initially identified for its role as an axon guidance molecule expressed during foetal development. It is normally turned off in adult organisms. Although Netrin-1 has been shown to be produced in a number of cancers – in particular those that have undergone metastasis – the mechanism for upregulation has not been unknown.
In the current study, Carpizo and colleagues delineated a mechanism involving extracellular vesicles budding off from pancreatic cancer cells, entering the blood stream, and becoming lodged in the liver. While extracellular vesicles contain Netrin-1 in the pancreas, a ‘feed-forward’ mechanism stimulates production to significantly escalate once they reach the liver. Here Netrin-1 activates hepatic stellate cells, changing them into myofibroblast-like hepatic stellate cells. There myofibroblast-like hepatic stellate cells then release retinoic acid, which in turn stimulates tumour cells to increase Netrin-1 expression, which signals through its receptor Unc5b to promote dissemination and metastatic tumour cell survival. In effect, Netrin-1 works to precondition the metastatic niche in the liver to receive the cancer cells.
“The discovery that Netrin-1-positive EVs [extracellular vesicles] activate HSCs [hepatic stellate cells] and contribute to liver metastasis through HSC activation indicates that EV-associated Netrin-1 is functionally relevant to liver metastasis,” write the authors.
In mouse models of pancreatic cancer, they went on to show that NP137, a first-in-class humanised monoclonal antibody directed against Netrin-1, was able to suppress metastasis and increase survival. “This provides the first pre-clinical evidence of the efficacy of anti-Netrin 1 therapy in pancreatic cancer,” says Carpizo. On the basis of these findings the team are now planning a pilot clinical trial, due to start at the end of 2023, where two cycles of NP137 will be given preoperatively to 25 patients with resectable PDAC. The primary endpoint will be a measurement of epithelial-to-mesenchymal transition, a biological process closely aligned with metastasis that has been shown to be inhibited by NP137 in other cancer types.
“There is likely to be a role for NP137 in all stages of pancreatic cancer, although we currently don’t know if it would be better at suppressing the growth of microscopic metastases or metastases that have already formed,” Carpizo tells Cancerworld. As yet, it is also unknown whether, in pancreatic cancer, NP137 should be used in combination with chemotherapy and/or immunotherapy and whether it could also be used to treat metastases from other tumours.