Could the frantic search for drugs to treat patients severely affected by the covid-19 virus be a shot in the arm for new cancer treatments? There have been many headlines about repurposed agents, such as the highly debated antimalarial drug hydroxychloroquine or tocilizumab, an anti-rheumatic drug targeting Il-6. Along with many others, the research to find effective treatments (and vaccines) has resulted in some of the most rapid scientific collaboration of recent years, compressed into just a few months.
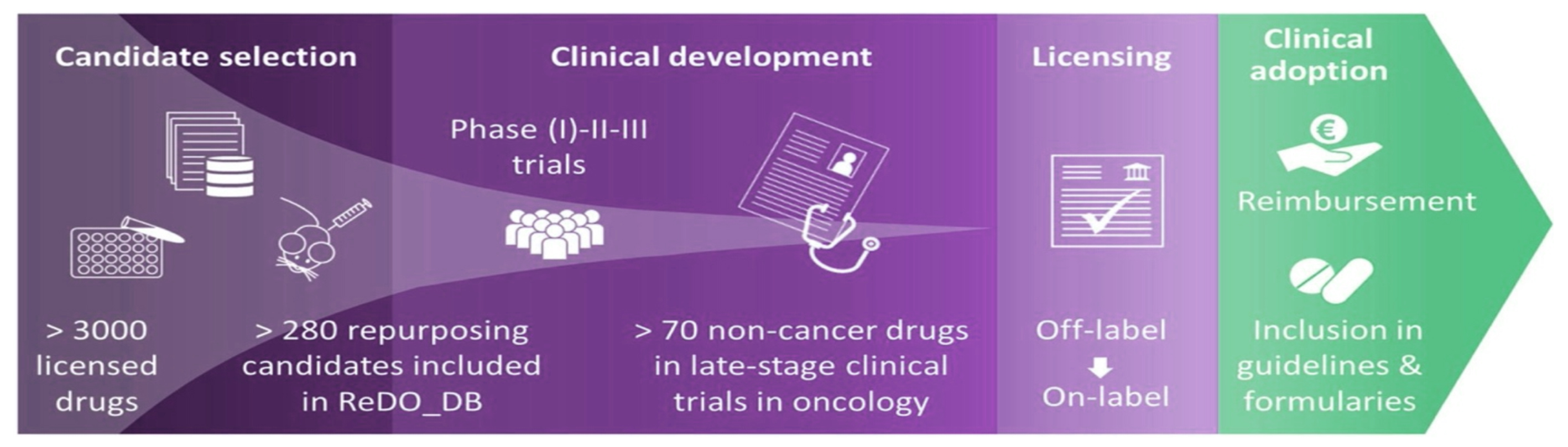
Drug repurposing – ‘new targets for old drugs’ – has been an active research field in cancer for many years, with many thousands of papers on numerous agents for treatment and prevention. The experience in oncology has informed the hunt for covid-19 drugs in terms of strategies and tools for treatments and vaccines, and some cancer drugs are themselves also candidates (Ciliberto G et al. J Exp Clin Cancer Res. 2020). A new field of computational drug repurposing (Park K Transl Clin Pharmacol 2019) has opened up in response to the high cost of drug development, and this approach is being applied to covid-19. But could covid-19 also spark more interest in speeding up new drugs for cancer, particularly in addressing regulatory and financial obstacles that stand in the way? Those barriers are significant because the burden of establishing new uses for old, off-patent agents that have low financial value resides mainly with organisations outside of big pharma, such as institutes, academia and healthcare payers, mostly in the non-profit sector. Industry has little interest in repurposing these drugs, especially for limited markets, such as rare and paediatric cancers. Nevertheless, the maze of trials, patents, marketing authorisations and product vigilance still has to be navigated, and few short cuts exist, although a key advantage is not starting from scratch because the drugs are already available and have been through pre-clinical testing for safety.
Covid-19 has created impetus to change the landscape of repurposing, as it has opened the door to billions of dollars of funding for treatment and vaccine research, with a major focus on repurposing drugs such as remdesivir, and the chemical entity hydroxychloroquine. Given the synergies with oncology repurposing, it is a good time to assess whether there is likely to be a boost for other fields, such as cancer, and a new opportunity to make changes to pathways to drug availability. This is what the Anticancer Fund, a Brussels-based advocate of repurposing, duly did in an online meeting in June, ‘Drug repurposing for cancer in the covid-19 era’.
Anticancer Fund ReDO
The Anticancer Fund is the home of the Repurposing Drugs in Oncology (ReDO) project, launched in 2014 with the remit to support repurposing well-known non-oncology drugs as cancer treatments. Its database, last updated in February 2020, lists 310 drugs that ‘warrant scientific investigation’ for their potential use in cancer, although the existing evidence for effects is ‘very limited’ for most agents. The database itemises existing main indications, whether a drug is on the WHO Essential Medicines list, if it is on or off patent, and what level of cancer-related research has so far been carried out (such as in-vitro, in-vivo, or human trials).
Of the two main categories for use of drugs in cancer – prevention and therapy – the majority of candidates are in the latter. Prevention drugs, which include preventing tumour recurrence, comprise aspirin, statins, the diabetic agent metformin, and selective oestrogen receptor modulators (SERMs). Two SERMS have been approved and are in clinical use for some time, namely tamoxifen and raloxifene. Most therapeutic investigations concern drugs used in cardiovascular conditions, which include beta-blockers; antipsychotics and antidepressants; antimicrobials, antibiotics and antivirals; and nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, celecoxib and ibuprofen.
Also under active research are drugs such as leflunomide, approved for rheumatoid arthritis, and thalidomide, a teratogenic compound, which has gained approval for the treatment of multiple myeloma, as well as the steroid dexamethasone, which coincidentally is one of the more promising treatment candidates in the fight against covid-19. Two examples of repurposed agents approved for oncology are arsenic trioxide and the acne drug all-trans retinoic acid (ATRA), both for leukaemia. The addition of non-oncology drugs to existing treatments is also a feature of many investigations owing to potential synergistic effects.
Indeed, most repurposing in oncology concerns new indications for existing cancer drugs. Pan Pantziarka and others who run the ReDO project describe this as ‘soft’ repurposing (Pantziarka P et al. Front Pharmacol 2018) rather than ‘hard’ repurposing for non-oncology drugs. But, while there are advantages in having extensive experience with drugs already approved in oncology, in the era of personalised medicine there has been limited progress in finding effective new indications for targeted drugs by trying to match them to patients with different tumour types and mutational profiles suggestive of benefit. Furthermore, most targeted agents and immunotherapies benefit only small subsets of patients with certain cancers.
An overlooked area is cytotoxic chemotherapies – while few new cytotoxics have been introduced in recent years, they are still a mainstay (Maldonado E et al. J Clin Oncol 2019) of adjuvant therapy and treatment for advanced cancer and the perceived reduction in use of cytotoxics is more hype than reality (Feinberg B et al. Am J Manag Care 2019). There is advocacy for doing more research on combinations with all types of agents, and research is identifying potential new uses. For example, pentostatin was found to work in a form of leukaemia other than originally proposed (finding uses for failed drugs is part of the repurposing picture). In proposing candidates for a repurposing pilot, the Anticancer Fund has included a chemotherapy drug (docetaxel) and also hormone (letrozole), as well as the osteoporosis drug (zoledronic acid) alongside non-oncology drugs.
The missing link?
But the array of non-oncology agents is regarded as the ‘missing’ link (Pantziarka P et al. Front Pharmacol 2018) in the current armoury of medical therapies for cancer, and some say it is even a ‘treasure trove’ that “should not be ignored since [the drugs] could target not only known but also hitherto unknown vulnerabilities of cancer”. This and other recent papers are characterising the spectrum of non-oncology drugs as potential candidates for combating most of the hallmarks of cancer. Papers tend to fall into two camps: those that review agents or groups of agents for potential, and those that take a certain cancer and discuss potential repurposed options, such as triple-negative breast cancer (Spini A et al. ecancer 2020).
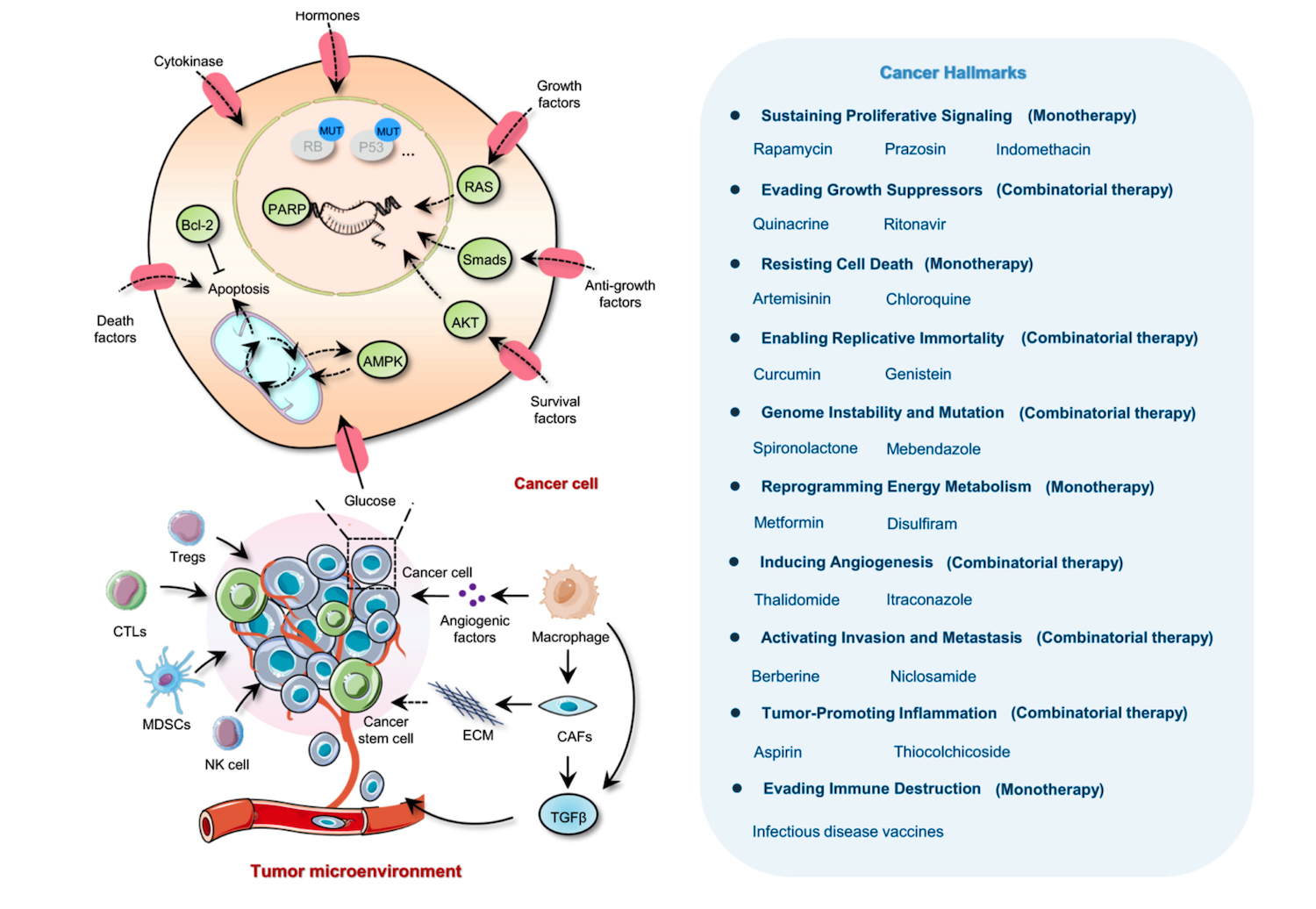
Figure redrawn based on the original article published by Zhang, Z., Zhou, L., Xie, N. et al. Overcoming cancer therapeutic bottleneck by drug repurposing. Sig Transduct Target Ther 5, 113 (2020). https://doi.org/10.1038/s41392-020-00213-8
While few of the candidates for hard repurposing have yet been approved for new indications in cancer, the sheer volume of investigational research is seductive, and shows no sign of waning, as illustrated by studies of acne, allergy and arthritis agents, to those for depression and diabetes, and parasitic infections and Parkinson’s disease. Are the barriers mainly organisational, which was mostly the focus of the Anticancer Fund webinar, or scientific, or more likely a combination?
Numerous phase III trials of approved cancer drugs have shown limited survival benefit, and some trials of repurposed drugs have mirrored these outcomes. For example, results recently reported of a trial of adding celecoxib, a cox-2 inhibitor used in arthritis, to an adjuvant (FOLFOX) regimen for colorectal cancer showed no disease-free survival benefit. Celecoxib has been studied preclincally and in trials of cancer prevention and treatment with promise in colorectal cancer, (Toloczko- Iwaniuk N et al. Curr Drug Targets 2019) so this result was disappointing.
There is solid evidence that Cox inhibitors, in particular aspirin, which is probably the exemplar drug in non-oncology agents, have anti-cancer properties. Analyses of its use in preventing heart attacks have shown preventive benefits in colorectal cancer, especially, which is well known. Despite this promise, Cox inhibitors can have side-effects, such as gastrointestinal bleeding with aspirin, and latest research indicates that aspirin may actually have an adverse effect for cancer among older people (McNeil JJ et al. J Natl Cancer Inst 2020). Moreover, an early prevention case-control study of Cox inhibitors proved to be cardiotoxic and lethal in a certain percentage of patients, which limited their use (Graham DJ et al. Lancet 2005). Indeed, it’s been said that aspirin would not be approved today owing to side-effects that would have been revealed in animal studies.
Nevertheless, there are many aspirin studies in both prevention and treatment, such as a basket trial, Add-Aspirin, which has resumed recruiting patients with colorectal, breast, gastro-oesophageal and prostate cancer. Evidence may establish aspirin as a standard of care, possibly through identifying only people who benefit (Coyle C et al. Curr Colorectal Cancer Rep 2016) rather than by a population approach, but there would appear to be little incentive for any marketing authorisation holder to seek a regulatory indication for one of the cheapest over the counter generic drugs. The same would apply to other agents such as high-dose vitamin C, which is the subject of renewed interest in cancer, and statins, but where such agents are shown to complement cancer drugs or radiotherapy, they may pave the way owing to the clout of a major oncology player.
“Crucially, the array of non-oncology agents is regarded as the ‘missing link’ in the current armoury of medical therapies for cancer”
Of course, there is nothing to stop oncologists prescribing medical therapies off-label, as numerous cancer drugs are prescribed this way, especially in advanced tumours in later lines when other therapies fail or for rare or paediatric cancers. Guidelines recommend off-label use – for example NICE, in its colorectal cancer guideline, says daily aspirin can be considered as a prevention drug in people with Lynch syndrome, but notes that as of January 2020 this was an off-label use.
The advocates of drug repurposing are aiming their sights much higher, as other drugs that lack any scrutiny by regulators for cancer indications will probably fail to be used widely owing to perceived lack of evidence and publicity. Not least, there is the biggest incentive of all at stake: money, with ‘prescription bias’ towards costly, well-marketed treatments. There are echoes with the controversy over the use of the much cheaper Avastin instead of Lucentis in macular degeneration, as both agents have a similar mechanism of action, and recognise the same target.
An example in oncology is in agents used to treat nausea and vomiting caused by chemotherapy. Costly antiemetic drugs with chemotherapy indications, such as aprepitant, are often used, but there is evidence that cheaper approaches, (Gyawali B et al. J Global Oncol 2015), including the drug olanzapine, an antipsychotic, are also effective, and could be offered to patients less able to afford treatment. Olanzapine is now appearing in guidelines.
“However, drugs that lack any scrutiny by regulators for cancer indications will probably fail to be used widely owing to perceived lack of evidence and publicity”
Where are the incentives?
At the Anticancer Fund webinar, attention turned to Hans-Georg Eichler, senior medical officer at the European Medicines Agency (EMA), who is engaged publicly and academically in regulatory policy. Eichler noted that if an academic group obtains good results for a repurposed drug they would have to approach existing holders of marketing authorisations, and there may be concerns that trials of these drugs are not good enough to meet regulatory standards. This may be because of lack of resources to obtain scientific advice on trial quality. Then, if a licence for a new indication is granted, there must be commitment to ongoing pharmacovigilance and post-authorisation studies. Notably, he confirmed a lack of incentives for a marketing authorisation holder if there is zero or close to no revenues from a new indication.
“If an academic group obtains good results for a repurposed drug they would have to approach existing holders of marketing authorisations”
Eichler said the EMA can offer informal advice and its hefty fees can be waived for scientific advice to academic groups developing orphan drugs. But, most licensing obligations and costs cannot be avoided and he urged that they be taken into account in grants given for drug development. He suggested that one way to cover all bases is to set up small spin-off businesses from universities (the EMA does offer help to SMEs), and also noted that a similar lack of incentives exist in other areas, such as developing new agents to combat antimicrobial resistance.
Clinicians presenting in the webinar said it is a struggle to get colleagues to take repurposed drugs seriously – most want to work on the ‘sexy’ targeted or immunotherapy drugs and not on drugs that are not studied primarily in cancer because they may have a perceived less well-defined target mechanism of action. It was mentioned that repurposed drugs seem to require a higher evidentiary standard than the expensive new agents trialled by industry, which mostly have low response rates, below 10%. Eichler countered that approvals may be given preferentially to drugs researched in small trials for life-threatening diseases where there is an unmet need and no other treatment options, but that does not indicate lower standards.
An example of a promising trial supported by the Anticancer Fund was given by Nicolas André, an oncologist at the Timone children’s hospital in Marseille. This phase I study in children with rare optic nerve glioma, tested a low-risk combination of an anti-inflammatory and a statin, instead of chemotherapy. André reported a 50% success rate in controlling disease after 6 months among participating centres in France, a standard that should be required for a new treatment strategy. But, at present, there are no partners on board to help take this work to the next phase.
Audrey Tran and Vinay Prasad (Tran AA & Prasad V. Lancet Oncol. 2020) have commented that repurposing efforts, while well intended might be misguided as the agents often lack single-agent activity. Overall, they state that alternatives exist that achieve the same goals as repurposing but are a better use of resources.
Bringing stakeholders together
The Anticancer Fund aims to bring stakeholders together to further repurposing research. Lydie Meheus, the fund’s managing director, also noted how researchers are dependent on engaging marketing authorisation holders with compelling clinical data. One promising avenue she pointed to is using the power of platforms to generate evidence for a range of agents at the same time, which is what the world-leading Recovery trial has done in the UK with covid-19 drugs. It compared several treatments with standard hospital care and found that dexamethasone, the low-cost steroid, reduced mortality in those receiving respiratory support, and found no benefit for hospitalised patients with covid who received hydroxychloroquine.
It’s an approach that could be used more widely in oncology she said, as it can eliminate futile interventions and focus on promising ones, and can also attract industry money for some investigational arms; platform trials are costly to run. An award-winning platform trial in the UK studying prostate cancer is Stampede, where industry and non-profit organisations have put agents into comparison arms. Stampede, which has been running since 2005, and is currently testing metformin among other agents, has also included the now off-patent chemotherapy, docetaxel. Another platform trial in the UK is Focus4, which includes an arm looking at a different way of using capecitabine, an off-patent chemotherapy drug, in colorectal cancer.
Innovative trial designs are a way to increase the chances of success with repurposing, but more incentives and funding are needed for independent research, and to encourage more collaboration among research institutions, industry and regulators. There are a number of barriers for certain agents (Pantziarka P et al. Semin Cancer Biol 2020) that show promise as repurposed options, and have yet to reach regulatory approval; these include nelfinavir, which is no longer marketed in Europe; propranolol, which has been reformulated for a childhood illness and given patent protection; clarithromycin, which has more than 200 marketing authorisations authorised by national procedures, which complicates label extensions; and auranofin, which is barely used and has been withdrawn from most markets, thereby hindering repurposing adoption.
STAMP brings a helping hand or Safe and Timely Access
Help is at hand from the European Commission’s expert group on Safe and Timely Access to Medicines for Patients (STAMP), which was set up in 2015. It has been piecing together a framework for how organisations could advance a repurposed drug over the regulatory hurdles. A draft, issued in March 2019, defines the components of repurposing projects. There should be a valid marketing authorisation holder in an EU member state or at EU level; the organisation driving the project is termed a ‘champion’; and the steps it should take include seeking scientific advice, and forming partnerships with marketing authorisation holders, which then take forward a regulatory dossier.
The Anticancer Fund has proposed a pilot comprising drugs that would test a number of scenarios in the framework, including ‘hard’ and ‘soft’ candidates; early versus late stage of development; national versus centralised approval – drugs developed to treat cancer must go through the EMA, but most others have only national approvals; and drug combinations versus monotherapy.
The challenge of keeping potential partners committed is exemplified by one trial, CUSP9, which has been investigating no fewer than nine repurposed drugs in combination with temozolomide for treating recurrent glioblastoma, and is a good test case for the framework of early-stage research. By contrast, docetaxel for hormone-sensitive prostate cancer, which was already used off-label and was a candidate in the Stampede trial, was discussed to test a late stage entry into the pathway. In fact, last autumn the EMA did extend the drug’s approval for this indication. The pilot though is currently on hold.
The EMA’s strategic reflection on regulatory science to 2025 commits the agency to supporting a repurposing framework (the EMA is a member of the STAMP expert group). There is little indication though that such regulatory issues will be taken on board in the latest EU cancer initiatives taking shape, namely the Mission on Cancer and Europe’s Beating Cancer Plan. There is reference in the interim report by the board of the Mission on Cancer to the burden of toxicity from using old off-patent, off-label drugs in treating childhood cancers, and lack of approvals of new paediatric agents.
The Covid effect
There is no doubt that Covid-19 (Saini KS et al. Br J Cancer 2020) pandemic itself is a pilot for possible change in how new drugs are assessed and authorised at a time when a number of traditional processes, such as site visits, were not possible. The wider agenda is also about the future of independent clinical research and strategies, such as treatment optimisation, (Beishon M CancerWorld 2020) for which reform of regulatory frameworks may be necessary.
The EMA is currently reviewing the results of the Recovery trial and the potential use of dexamethasone, following discussion by the agency’s Covid-19 task force. Dexamethasone is an inexpensive, off-patent drug that is authorised in the EU by national medicines authorities. The trial has been praised for its speed, scale and transparency.
As Martin Landray (Wise J & Coombes R BMJ 2020), one of the investigators in the Recovery trial, has said: “How can we build on the involvement of patients and clinicians and the timely access to relevant data? We now need to apply the lessons from this approach to other major health challenges such as heart disease, cancer, arthritis and mental health.”