Posts by tag
drug development
Why Patient-Reported Outcomes are Rarely Used in Trials, and How We Change That
If drug developers design trials to measure how well their drug addresses the issues most important to the target patient community, we could expect to end up with better drugs. Integrating patient-reported outcomes into clinical trials is the way to…
The CRISPR revolution: it’s transforming cancer research, can it do the same for treatment?
CRISPR is a revolutionary gene-editing tool that allows scientists to cut DNA with extraordinary precision and make changes to the genome. The technology was a gift to cancer researchers, whose efforts to understand the roles played by different genes rely…
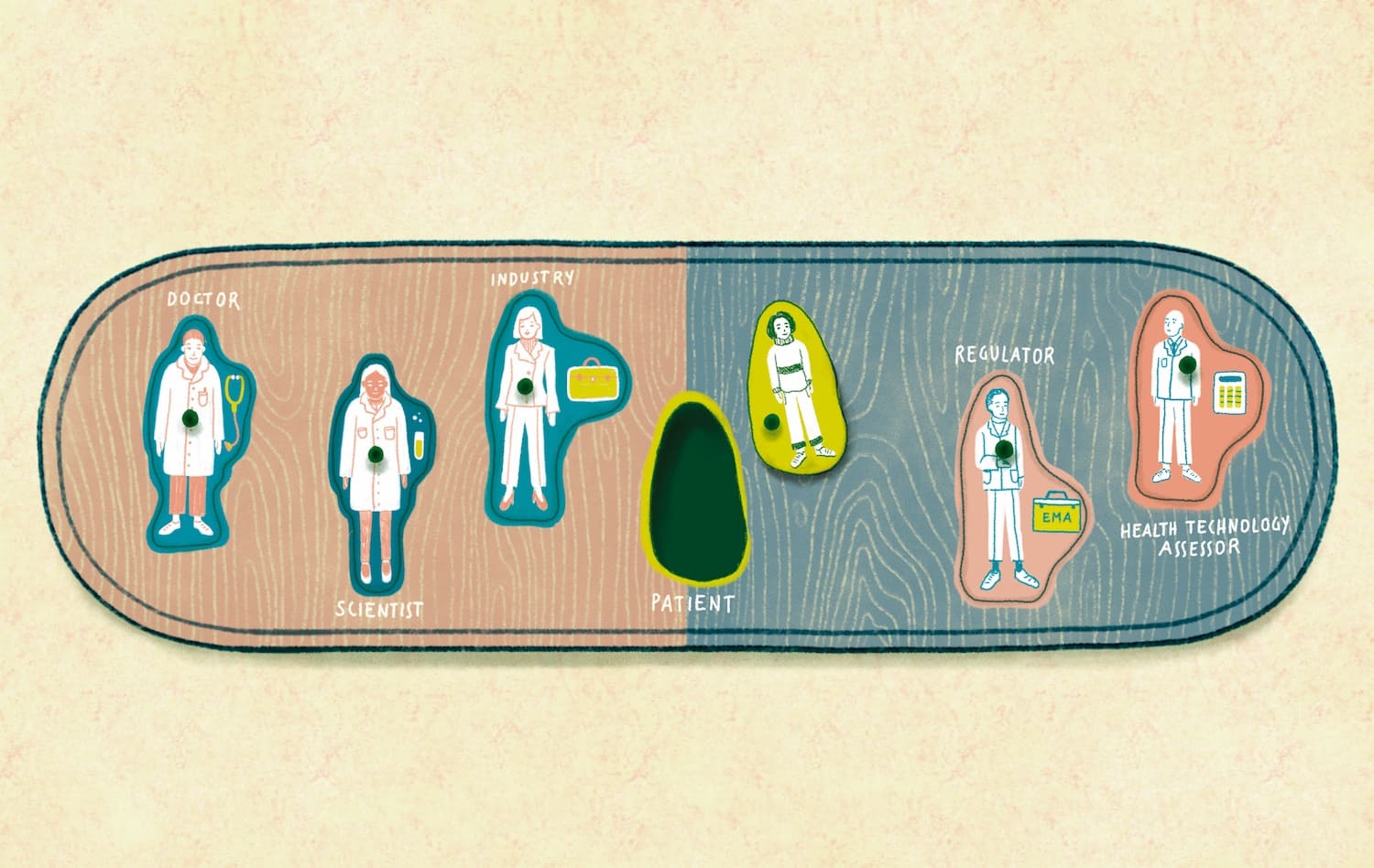
EMA at 25: learning more from cancer patients
Responsibility for scientific evaluation, supervision and safety monitoring of medicines was done at the national level until 1995, when EU member states agreed to coordinate that work within the European Medicines Agency (EMA). A quarter of a century on, and…