Bone metastases occur in around 7 out of every 10 people with advanced cancer, and are most frequently found in people with breast, prostate, lung or kidney cancers, and in most people with advanced multiple myeloma. They cause pain and raise the risk of bone fractures, spinal-cord compression and other serious conditions, and also cause anaemia and excess calcium (hypercalcaemia). While gaps remain in our understanding of their biology, they can often be managed easily and effectively. Yet across Europe, people are continuing to suffer unnecessary pain and complications caused by poorly controlled bone metastases. Improving access to the right treatment at the right time could make an important contribution to the quality of life of the growing number of patients living many years with advanced cancers.
Understanding bone metastases
Early thinking about why breast and prostate cancers often spreads to bones, in particular along the spine and to appended structures, focused on the proximity of the vertebral venous system. That is now understood to be an insufficient explanation, says Peter Hoskin a clinical oncologist at the UK’s Mount Vernon Cancer Centre and oncology professor at the University of Manchester, who counts palliative radiotherapy among his research interests and has played a leading role in education at both the UK and European level through ESTRO, the radiotherapy society. “We know a lot more about how and why a cancer cell homes into a particular environment – about the biochemical properties of cells and the pathophysiology of bone metastasis,” Hoskin says. The developing knowledge, he says, builds on the ‘seed and soil’ hypothesis, but remains incomplete, as bone metastasis is a highly complex process that is not yet fully understood. The crucial process, explains Hoskin, is the activation of osteoclasts, which cause the reabsorption of bone, through various factors. This action upsets the normal bone equilibrium between reabsorption and growth, and leads to a vicious cycle of bone destruction and tumour growth. There are two main types of bone mets − ‘osteolytic’ mets (destruction of bone caused by osteoclast activation) and ‘osteoblastic’, sclerotic overgrowths, where the reverse happens and bone growth predominates. Different cancers predispose to different types of bone met: kidney and multiple myeloma, for instance, tend to form predominantly osteolytic mets, while osteoblastic mets tend to predominate in metastatic prostate cancer. Both processes, however, may be in play, and mixed lesions are seen, particularly in breast cancer. Hoskin adds: “I don’t think we fully understand why this happens – we know the mechanisms involved but not the full cascade of factors that causes the imbalances” (Coleman RE et al. Nat Rev Dis Primers, 2020). Hoskin says that about 70% of metastatic breast and prostate patients have bone mets, but he notes that there has been a stage shift in prostate cancer owing to earlier detection, and the introduction of bone targeting agents, principally bisphosphonates, have also had a big impact on the pattern of bone mets, especially in breast cancer.

Managing bone mets
Bisphosphonates help prevent and treat bone mets; they target the osteoclast pathway, and include zoledronic acid. The monoclonal antibody, denosumab, is a more costly and relatively novel alternative, with other agents in trials (Coleman RE et al. Nat Rev Dis Primers, 2020; Wang M et al. Bone Res, 2020). But treatment for bone mets is multimodal and multidisciplinary. External-beam radiotherapy is a mainstay for palliative care of bone mets, as well as bisphosphonates. Treatment options also include surgery, standard analgesics, cancer-directed medical therapies, radiopharmaceuticals such as radium 233 – which can target osteoblastic bone mets – and treatments for hypercalcaemia and other symptoms of bone mets besides pain. Imaging plays a big role in diagnosis and treatment monitoring (Coleman RE et al. Nat Rev Dis Primers, 2020). As Hoskin also notes, there is growing evidence for targeting the few mets (including bone) in oligometastatic spread, such as in prostate cancer, which crosses over into potentially curative or long-term chronic management territory. For developments in oligometastasis see: Beishon M. Cancer World, 2018. An important point, he adds, is maintaining quality of life and possibly postponing interventions that diminish quality further, such as androgen-deprivation therapy in prostate cancer.
When it comes to managing bone mets pain, external-beam radiotherapy remains a pivotal treatment. “Palliative radiotherapy of bone mets is a gold standard and has been for a long time,” says Hoskin. “A low dose of radiation gives good pain relief – we don’t give high doses like we do to primary tumours. We can see some tumour shrinkage, suppression of osteoclast activity, impact on nerves and other effects. We don’t really understand why it works but it does manipulate the microenvironment of the bone, such as by encouraging the osteoblastic activity of the bone to be dominant rather than suppressed.”
“Palliative radiotherapy of bone mets is a gold standard and has been for a long time”
Palliative radiotherapy can give a complete response to pain in up to one third of patients and a higher number of partial responses, which can last for the entire course of their disease, although re-treatment may be necessary. It is also used to treat fractures and high-risk lesions that may fracture, such as in the load-bearing femur, and the distressing and dangerous effects of spinal-cord compression where the disease is in the vertebrae. It is an effective tool for preserving quality of life in the growing number of people who live with metastatic cancer for a long time. Yet for many, even in countries with adequate radiotherapy capacity, access is a problem, with people often having to wait for several weeks for relief from this tried and tested intervention, and others never receiving such treatment at all, as palliative radiotherapy – and radiotherapy in general – are undervalued and underused.
Improving access
There would seem to be a simple answer: the rapid access palliative radiotherapy service. This is a dedicated resource to speed up palliative treatments to overcome unacceptable wait times. Such programmes exist – they appear to have been pioneered in Canada in the mid-1990s, according to a timely review paper by Kristopher Dennis and colleagues published this summer, who searched for published evidence on features and outcomes globally (Dennis K et al. Clin Oncol 2020). The authors found 19 publications, with eight from Canada and one or two from Australia, India, Ireland, Italy, New Zealand, the UK and the US. Apart from speeding up access to care, these services can facilitate multidisciplinary care for people with advanced disease, who need more complex attention than most. They also enable the development of advanced roles for staff, such as radiographers (Fessl S. CancerWorld, 2020). They help to develop and refine the evidence base, such as for the delivery of single rather than multiple fractions of radiation, and for new technologies and procedures. Frail patients and those who may have to travel long distances may derive particular benefit from having the chance to be seen quickly with a limited hospital stay. One extreme example is Inuit patients in Canada who face a 2,000 km flight to be seen. Another paper led by Dennis details experience with rapid access programmes and how they serve as models for multidisciplinary care and education (Dennis K et al. Future Oncol, 2015). One of the publications in the 2020 review paper is by Linda Bedford, a consultant radiographer who runs the palliative radiotherapy service at Musgrove Park Hospital, in Taunton, UK. She says the previous radiotherapy manager identified gaps in services that led to the appointment of two consultant radiographers – one for palliative radiotherapy, the other for late-effects of radiotherapy – and the two posts were initially funded for three years by Macmillan, the cancer charity. Bedford took the palliative post. “It was a blank canvas – there was only one other such service in England,” she says. “The standard process had been for any patient requiring radiotherapy to be seen by clinical oncologists according to specialty such as prostate or breast. However, the wait times for palliative treatment could be as long as two weeks,” says Bedford, who says caring for people living with advanced cancer is “a passion of mine”. “Generally there can be a view that ‘palliative is only palliative’, but as it is about 40−50% of the radiotherapy caseload there is a lot of unmet need if people have to wait, especially with patients living with non-curable cancers for a long time,” says Bedford, who adds that NHS England has added cancer to its long-term conditions compendium, an important step towards improving services for people with advanced cancers.
“Generally there can be a view that…‘palliative is only palliative’, but… there is a lot of unmet need”
Bedford has the authority to assess patients and plan and prescribe certain radiotherapy treatments and drugs without review by a clinical oncologist; treatment is then delivered by a radiography team. Radiographers in the UK, as in most countries, are not medical doctors and the governance for such authority has to be defined within strict training and competency parameters. But although the NHS has pioneered advanced practitioner roles for a number of groups in the allied health professional category, it is usually the responsibility of local providers to work out the protocols. Given the pressure on resources and shortages of personnel, expanding the boundaries for allied health staff is logical but varies greatly around Europe and there may be opposition from doctors. Bedford says the oncologists at Taunton are only too happy with the consultant radiographer roles, which are now funded by the hospital trust. She says the patient pathway has been speeded up significantly. “Patients are referred to me in several ways – they can do so themselves if they are struggling at home and their next appointment is some way off; they may come into the hospital because of pain; or their GP may call. No matter how, I have flexible clinics and try and set aside time to see them every day – and we have cut waiting times to be then referred for radiotherapy from 13 days by oncologists down to three days for me.
“We have cut waiting times to be then referred for radiotherapy from 13 days by oncologists down to three days for me”
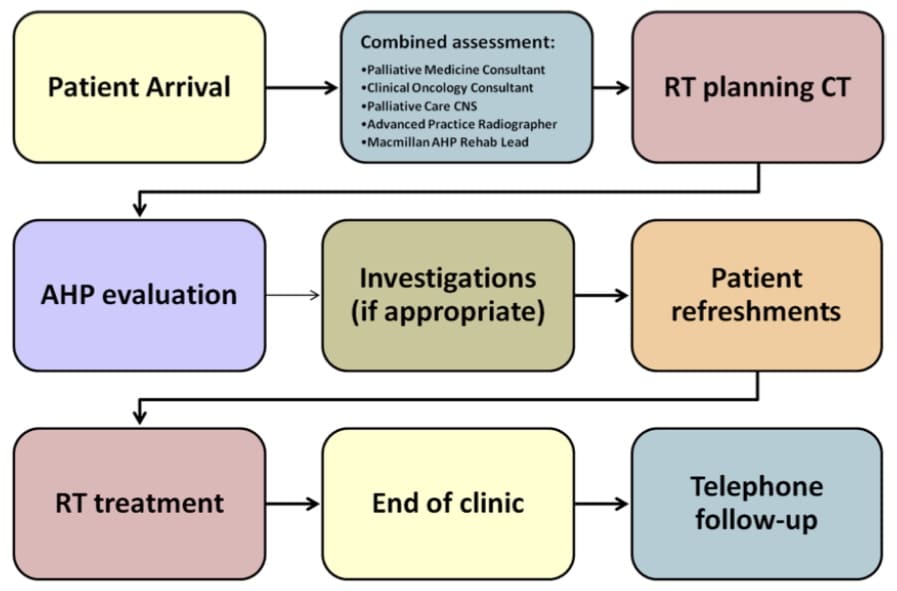
“In the palliative world we have two main pathways – an emergency, such as for a spinal cord compression, which is on a 24-hour pathway, or a 14-day one, such as for pain, to be started within that time. But much of that is pushed back to the end of two weeks.” The patient mix at Taunton mirrors the expected distribution of bone metastases – about 80% are prostate, breast, lung and myeloma cases; 20% the rest. Bedford is also qualified to deliver whole-brain radiation therapy, but the evidence now indicates less frequent use. She has achieved what she sees as an intermediate pathway – and it is certainly possible to go further and complete a consultation, scan and treatment on the same day, as well as address other needs. This is being done at University Hospital Southampton, also in the UK, in the Rapid Access Multidisciplinary Palliative Assessment and Radiotherapy Treatment (RAMPART) clinic. It is aimed at patients with bone mets, and a report on the service notes that a team has managed to do in one half-day visit what used to take 2−3 weeks and at least three appointments.
A team has managed to do in one half-day visit what used to take 2−3 weeks and at least three appointments
The RAMPART clinic is led by clinical oncologist, Paul Fenton, and patients receive assessments from oncology, radiotherapy and palliative medicine in collaboration with occupational therapy and dietetics during the visit. Data collected by the team has shown that more than three-quarters of those who attended the clinic had occupational therapy and dietetic needs, with the most frequent concerns relating to pain (72%), fatigue (55%), changes in appetite and weight (40%) and breathing difficulties (31%).
Overcoming barriers
As Hoskin comments, this type of clinic demands a team and resources that are in short supply in many centres, although the intermediate stage should be achievable, particularly if more consultant radiographers can take a lead. As Bedford adds, patients need time to be assessed and express their concerns. “My post means I can give patients the extra time they wouldn’t have had before,” she says, noting that during the Covid-19 pandemic it has been possible to hold some consultations by video to speed things up. Pain itself is a big and often poorly understood subject and hard to measure, and patients can vary greatly in how they report it. The location of pain is also not predictable – “When we see mets on scans you would think those in a load-bearing bone would hurt most but often patients say pain is in peripheral bones, such as the ribs – we don’t know why this is,” says Hoskin. He also notes that it is hard to attract oncologists to palliative care and few would profess to be experts in pain, which is another reason why specialist teams are important. See also ESMO’s clinical guidelines on cancer pain management, on which Hoskin is a co-author.
“It is hard to attract oncologists to palliative care and few would profess to be experts in pain”
Other obstacles concern radiotherapy fractionation. Despite a solid evidence base showing that a single fraction can deliver the same pain control as multiple fractions, a majority of centres globally are still doing the latter, reckons Hoskin (Chow, R et al. Radiother Oncol, 2019). This may just be cultural inertia and not being a priority, but in some countries such as the US there are financial incentives to deliver more doses over several visits, and other countries may look to the US as a model. This is not good for people who may have to travel while in pain, and may also result in more expensive hospital care if patients have to be admitted. In a study led by Hoskin, the authors argue strongly for single doses to be widely promoted, with particular global benefits where resources are scarce (Hoskin P et al. Radiother Oncol, 2015). The lack of radiotherapy equipment in low and medium resource countries is a big issue that Hoskin highlights, and in Africa and elsewhere he says it may only be possible to give second-best drug treatments rather than the gold standard of radiotherapy. A study published in 2020 (Lievens, Y et al. Mol Oncol, 2020) shows access is an issue across Europe as well, with around one in four cancer patients who could benefit from radiotherapy not getting the treatment. The upshot is that this is a familiar story in oncology. There is a lack of good patient management that delivers a timely standard of care in many places and too few professionals to push for change. There are wide inequalities in resources. But there is also a lot of research that still needs to be done on understanding metastatic mechanisms, pain and other symptoms; optimising treatments; and developing new technologies and interventions. There is a big and expanding literature on bone metastasis, and answers to pressing clinical issues may come from the multidisciplinary palliative community. That last one may bring more resources – rather than being just a reactive service, there are new recommendations in the NHS to treat oligometastatic patients using stereotactic body (ablative) radiotherapy, and Bedford say she, for one, is ready to raise her game to accommodate such moves.
The development of osteoclastic and osteoblastic bone metastases

Source: Wang M et al (2020) Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res 8, 30 © 2020, The Authors. Reprinted under a Creative Commons Licence.
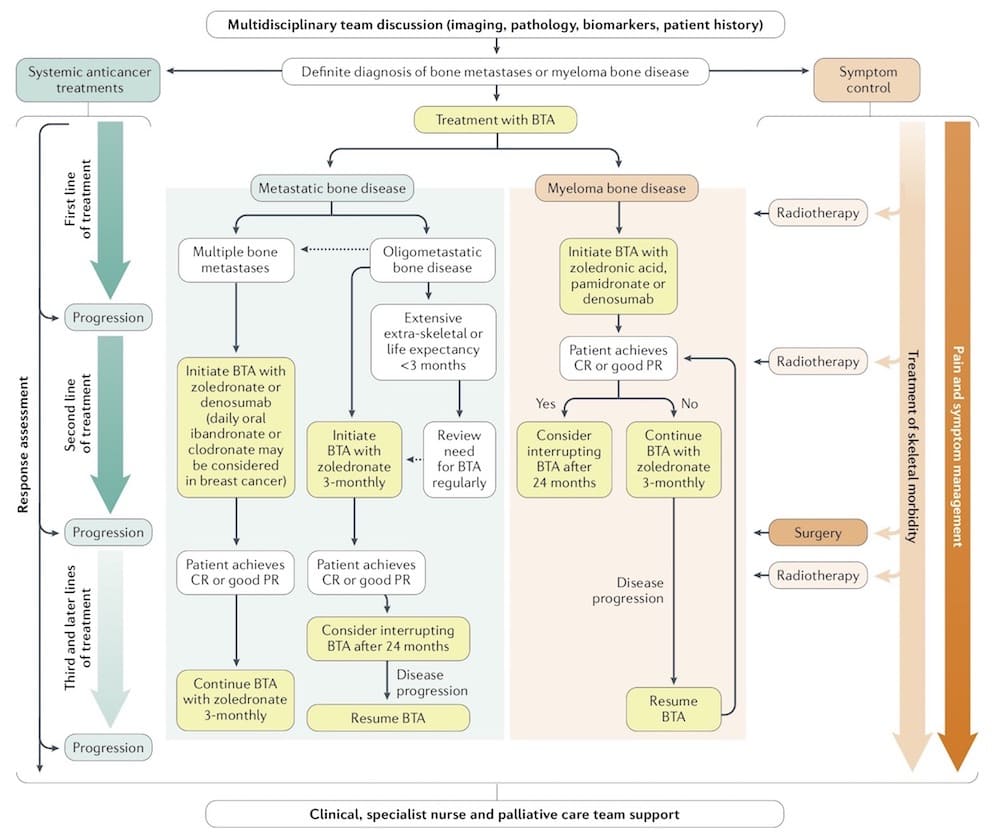
Schematic algorithm for multidisciplinary treatment of bone metastases and myeloma bone disease

BTA ‒ bone-targeted agent; CR ‒ complete remission; PR ‒ partial remission.
Source: RE Coleman et al. (2020) Bone metastases. Nat Rev Dis Primers 6, 83 © 2020 Springer Nature Ltd. Reprinted with permission
The RAMPART clinic model

Source: University Hospital Southampton NHS Foundation Trust (2017) Innovating for Improvement Round 2: final report Rapid Access Multidisciplinary Palliative Assessment and Radiotherapy Treatment (RAMPART) clinic. https://www.health.org.uk/sites/default/files/4.%20Uni%20Hospital%20Southampton_RAMPART_0.pdf Accessed 17 November 2020












