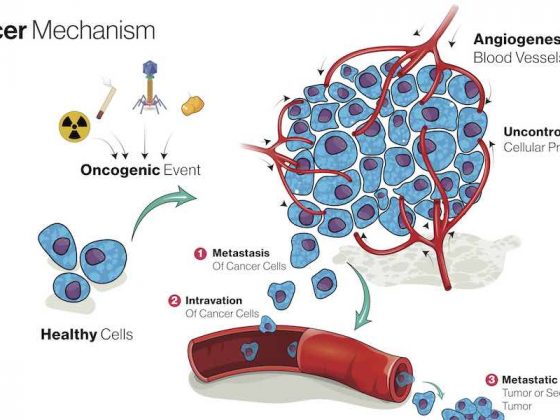
The vision of harnessing our immune systems to fight cancer has been tantalising scientists and doctors for more than a century. The idea had a strong scientific rationale: over millions of years our immune systems have evolved intricate and multi-layered ways to protect our health – why should cancer cells be exempt from its attentions? It was also seductive, offering the prospect of a more benign and more effective alternative to existing treatment options.
Yet for many decades, sustained efforts to assist and cajole our immune systems into action against cancer delivered one disappointment after another. The discovery that the CTLA-4 and PD1 proteins act as checkpoints that protect cancer cells from immune attack, and the idea – pioneered by Jim Allison – that the immune system could be freed to target cancer by blocking such immune checkpoints, provided the key that has unlocked progress.
The impressive results shown by the first CTLA-4 and PD-1 blockade therapies, in achieving complete remission in certain cancer patients with previously incurable stage IV melanomas – fuelled great optimism that this strategy could be made to work in a wider group of patients and a wider range of cancer types.
It also reignited hopes of making progress with the many other approaches to mobilising our immune systems against cancer that had been flagging for lack of success, including the use of oncolytic viruses and cancer vaccines.
These efforts received a second shot in the arm with parallel developments in gene editing technologies. Doudna and Charpentier’s development of the CRISPR/cas9 technique, which started to be used around 10 years ago, opened up mind-boggling possibilities for intervening in the behaviour of both cancer cells and immune cells. In the field of oncology, this approach was pioneered with CAR T cell therapy, which harvested T cells from patients and engineered them to recognise and attack their cancer cells. Since its first approval in 2017, CAR T cell therapies have been approved for treatment of a variety of blood cancers.
Both advances have truly transformed the prospects of many thousands of patients. And yet, as is so often the way in cancer research, generalising their success beyond the still limited settings where they work well remains a challenge.
Over the past couple of years, Cancerworld has spoken to many researchers immersed in efforts to do just that ‒ to translate our expanding knowledge about cancer and the immune system, and make use of our powerful tools of bioengineering, to find new strategies to get better results for wider groups of patients. We thought it could be an interesting exercise to review those stories to offer at a least a partial overview of where the science is going, and when we can expect results.
Oncolytic viruses
In December 2020, Cancerworld reported on developments in the use of oncolytic viruses to fight cancers. ‘Oncolytic viruses’ ‒ viruses that preferentially infect and kill cancer cells ‒ have been known about for some time. The first case report linking viral infection with the regression of an active haematological malignancy was published more than 120 years ago, sparking an interest in the possible use of viruses to treat cancer.
Progress with this strategy was seriously underwhelming until the turn of the millennium and has featured some false starts. RIGVIR, a non-pathogenic enterovirus, was approved in Latvia in 2004 for the treatment of melanoma, but was withdrawn in 2019 for lack of evidence. In 2006 the oncolytic H101 adenovirus was approved in China for the treatment of head and neck cancer, but that approval has not been extended to other regulatory bodies so far.
To date, the only oncolytic virus approved by the US and European regulators, FDA and EMA, is Amgen’s Talimogene laherparepvec (T-Vec or Imlygic) a modified herpes simplex virus approved in 2015 for subsets of patients with melanoma. Currently there are around 40 clinical trials of oncolytic viruses underway.
Key to making progress has been a better understanding of the mechanisms that link oncolytic viruses to cancer regression. Early hypotheses focused principally on the observation that certain viruses seem to prefer cancer cells, which is assumed to be linked to the changed metabolic status of cancer cells that makes viral replication favourable. The lysis of those cancer cells – i.e. the disintegration of the cells by rupture of the cell membrane – was seen as a straightforward consequence of those high concentrations of replicating virus loads. More recently, however, attention has focused on what happens next, in particular the cascade of signalling set off by the lysis, which alerts the immune system to a problem and calls it into action.
“It is the ability to rationally engineer viruses that can deliver immunotherapies that will provide the significant therapeutic impact”
Emerging technologies for gene editing are also opening up possibilities to tweak viruses, to target them at cancer cells, avoiding the need to attenuate them to avoid infecting healthy cells. They are also offering opportunities for amplifying the alert signals infected cells deliver to the immune system, and even for delivering cytotoxic chemicals to the heart of infected cancer cells.
Cancerworld talked to some of the people now trialling fascinating approaches that combine a range of strategies to ensure precise targeting of cancer cells with coding for expression within cancer cells that bring about their destruction in a variety of ways. These include flagging the cells to the immune system by coding for the expression of granulocyte-macrophage colony-stimulating factor (GM-CSF); removing the cancer cells’ protection from immune attack by coding for expression of an anti-CTLA-4 antibody, or delivering a cytotoxic agent directly to cancer cells by coding for expression of an enzyme that can convert the antifungal drug 5-fluorocytosine into the cytotoxic agent 5-fluorouracil.
Clinical trials have been initiated in a number of cancer types including advanced pancreatic cancer, melanoma, and metastatic colorectal cancer, cutaneous squamous cell carcinoma and anti-PD1 refractory melanoma.
As summed up by one of the experts, “I think we are moving from a vision where viruses would be used mostly for their [inherent] oncolytic properties to an era where they will be used as drug delivery systems… It is the ability to rationally engineer viruses that can deliver any number of immunotherapies that will ultimately provide the significant therapeutic impact.” This is definitely one to keep an eye on.
Gene therapies
In a separate article Cancerworld took a look at the whole field of gene therapies for solid tumours. Gene therapy involves manipulating or modifying the genetic make up of living cells for therapeutic purposes. CAR-T cell therapy, currently used to great effect for treating a variety of blood cancers, is a form of gene therapy, which counters cancer cells’ ability to hide from the body’s immune system by genetically manipulating patients’ T cells to recognise antigens specific to the cancer cells and attack them.
Decades of efforts to use gene therapy against solid tumours, in contrast, have yielded frustratingly slow progress. But as Cancerworld reported, in March 2021, that work may finally be beginning to bear fruit, offering the prospect of adding an additional therapeutic strategy to the oncologists’ armoury.
The biggest challenge is how to deliver the gene therapy exclusively to where it is needed without causing potentially fatal toxicities
We spoke to labs and biotechs trialling a variety of approaches. Some aim to assist the body’s immune system to swing into action against the cancer, for instance by delivering genes that code for interleukin-12 or interferon alpha ‒ both important activators of T-cells and natural killer (NK) cells. Others seek to use the so-called ‘suicide gene’ strategy that delivers to cancer cells a gene that codes for an enzyme that turns an otherwise fairly harmless drug into a powerful cytotoxic.
The biggest challenge continues to be around how to deliver the gene therapy exclusively to where it is needed without causing potentially fatal toxicities. Here again, Cancerworld was told of a variety of ingenious strategies, including using retroviral and lentiviral vectors, nanoparticles and even an electrotransfer method used by research laboratories to transfer DNA directly into cells.
The closest any of this has come to being approved in the US or Europe is an experimental gene therapy for treating high-risk non-muscle-invasive bladder cancer unresponsive to BCG therapy, which is currently under consideration by the US regulator, the FDA. Arguably this is low-hanging fruit because of the relative ease of delivery. The treatment, which uses a non-replicating adenovirus to deliver copies of the human interferon alpha-2b gene, can be delivered into the bladder where it comes into direct contact with the urothelial cells that need treatment, and can be contained in that part of the body.
The experts in the field stressed to Cancerworld that “great strides” have already been made in overcoming barriers that have dogged the development of gene therapy for solid cancers. They remain confident that it will find a role in the treatment of at least some solid tumours in the not-so-distant future.
Natural killer cells
In February 2021, our editor Adriana Albini took a look at progress in strategies to recruit natural killer (NK) cells into ‘a new tactical unit’ that could contribute their specific find-and-destroy skills to the anti-cancer immunotherapy brigade.
As Albini explained, NK cells have an advantage over T cells when it comes to detecting cancer ‒ they aren’t fooled so easily. While cancer cells have devised ways to hide from T cells the antigens that could reveal them as dangerous, NK cells are programmed to check identity cards, in the form of MHC class 1 molecules, and will destroy cells ‒ including cancer cells ‒ that cannot clearly demonstrate that they belong. NK cells are also able to spot some antigens on the surface of cancer cells that are not visible to T cells ‒ and they have some frighteningly effective weapons of extermination.
NK cells have an advantage over T cells when it comes to detecting cancer ‒ they aren’t fooled so easily
These tactical skills have excited the interest of cancer researchers for many years but, for a long time, efforts to weaponise them against cancer made little headway. A major barrier has been the raft of inhibitory receptors on the surface of NK cells designed to rein in their impressive killing powers, which include not only the now-familiar PD1 checkpoint but many others, some of which have only recently come to light.
This is opening the way for strategies involving developing drugs to inhibit these receptors to unleash the NK cells to do their job. Efforts are also underway to develop CAR NK cell therapy, which would work on similar lines to CAR T cell therapy. With more than 20 trials of this approach to treating solid as well as haematological tumours listed on clinicaltrials.gov in March 2022, it is not unreasonable to expect some of this to begin to make its way into the clinic in the not-too-distant future.
mRNA vaccines
Still in the field of bioengineering and harnessing the immune system, in May 2022 Cancerworld turned its attention to mRNA vaccines ‒ a technology that had been in development for use against cancer for 20 years before it finally shot to prominence during the Covid pandemic.
Here again we have a strategy whose potential as a cancer therapeutic has been suspected for many decades, but has struggled to deliver convincing results. The idea is to develop vaccines that do for cancer cells what traditional vaccines do for infectious pathogens, which is to teach the immune system to recognise ‘the enemy’ and build up its capacity to target and eliminate the threat.
Efforts to achieve this using traditional vaccines technologies had some small success in 2010, with the approval of Provenge to treat men with prostate cancer. The optimism that triggered about the potential for therapeutic vaccines against cancer was reflected in a Cancerworld article published in the same year, which talked about previous “false dawns”, and asked the question: Could compounds that teach the immune system to fight tumours be about to achieve their promise?
mRNA vaccine technology is now seen as a likely game changer because it can achieve the same end very much faster and easier
With hindsight, that optimism turned out to be a little premature. Provenge has not been a great commercial success, and was withdrawn from the European market in 2016 at the request of the manufacturer. That vaccine has to be developed individually for each patient, in vitro, harvesting their own dendritic cells and other antigen presenting cells and exposing them to proteins mimicking those found exclusively on their own cancer cells, in an attempt to stimulate and target them towards the cancer cells once reintroduced into the patient’s body.
mRNA vaccine technology is now seen as a likely game changer because it can achieve the same end very much faster, easier and more precisely by administering, in vivo, ‘messenger’ RNA that has been genetically engineered with instructions to build proteins that code for the production of the desired antigens.
Germany has been a pioneer in this field, and Cancerworld talked to some of those leading research efforts that have now reached the point of multiple clinical trials using a variety of approaches across a broad range of cancers and settings (advanced and adjuvant). These include efforts to develop “off-the-shelf” cancer vaccines that don’t need to be developed separately for each individual patient, and a fascinating approach to using vaccines to pre-empt resistance to treatment, by equipping the immune system to target common mutations known to confer resistance before they even occur.
As they point out, these efforts were already well advanced by the time pandemic came along, and interest and investment in the field has shot up as a result of the success of using mRNA vaccine technology against Covid. This is an approach we are likely to hear more about in the near future, though proving value in an adjuvant setting will always take time.
CAR-T against solid tumours
Then in April 2022 Cancerworld reported a news story from the Annual Meeting of the American Association of Cancer Research, which indicated possible progress in the elusive strategy of using CAR T cells against solid tumours. Like most news stories, it focused on the latest findings from one particular study, and did not seek to cover the rich variety of efforts in this field. It was interesting nonetheless for demonstrating some progress in addressing two longstanding challenges.
The therapy in question, BNT211, uses a novel target, the Claudin-6 antigen, which is known to be upregulated in at least 20 types of human cancer, including testicular, ovarian and gastric cancer and sarcoma, and is silent in healthy tissue. Finding such a target, that reliably distinguishes cancerous cells from healthy cells, has been one of the stumbling blocks for developing CAR T therapy against solid tumours. If this works out, it opens the potential for an ‘off the shelf” (‘one-size-fits-all’ or at least ‘one-size-fits-many’) treatment, which is far more efficient than having to develop CAR T therapies for individual patients using neoantigens specific to their personal tumour ‒ still the dominant approach for CAR T therapies used against blood cancers.
The second challenge it tackled was improving the performance of CAR T cells against solid tumours – getting them to do what they do, only better. This whole strategy is being developed by BioNTech, one of the German pioneers of mRNA vaccines. They have added to their Claudin-6-targeted CAR T cell therapy a novel RNA vaccine that amplifies CAR T cells to improve their persistence and functionality.
As our somewhat optimistic 2010 article on therapeutic cancer vaccines shows, it is difficult to predict quite where experimental strategies are going, or how long they will take to get there. But it is surely reasonable to hope that these many and varied efforts to equip the immune system to work against a wider range of cancers and benefit a wider group of patients will begin to pay off in the not-too-distant future.