August 7th 2020, much of the world was in various states of lockdown, anxiously awaiting news about progress in development of vaccines against the new SARS-Cov-2 virus, which by then had taken the lives of almost 1 million people. But it was in relation to a different virus that history was quietly being made, as the World Health Assembly adopted the first strategy ever aimed at the global elimination of a major cancer killer.
Cervical cancer, which kills more than 300,000 women worldwide every year, is caused ‒ in almost all cases ‒ by chronic infection with certain strains of the HPV virus. The global strategy aims to eliminate that cancer, along with other HPV-associated cancers, which include a high proportion of cancers at the back of the throat, as well as most anal, vaginal, vulvar and penile cancers. If successful, the long term impact could be massive, saving an estimated 62 million women from premature death over the course of a century, according to Karen Canfell, epidemiologist and health researcher at the Daffodil Centre (Sydney) and co-leader of the WHO Cervical Cancer Elimination Modelling Consortium.
How it will work: strategy
According to the WHO, eliminating cervical cancer requires that countries achieve and maintain an incidence at or below four cases per 100,000 women. The strategy to reach this goal involves a combination of prevention, screening and treatment. By 2030, 90% of girls should be fully vaccinated with the HPV vaccine by age 15; 70% of women should be screened by age 35 and again by age 45; and 90% of women with pre-cancer should treated and 90% of women with invasive cancer should be appropriately treated, managed and supported. The call from the World Health Assembly is for each country to aim to meet these ‘90-70-90’ goals by the 2030 deadline, in order to eliminate cervical cancer over the next century.
“These are all three sets of proven interventions, that target different parts of the life cycle,” says Canfell, who together with her research team supported the WHO in developing the strategy. “If we could succeed in scaling all of them up to a higher level, that would really set us on a path for cervical cancer elimination.”
Can it work? The example of Australia
As technologies and strategies for controlling HPV-associated cancers have developed over recent decades, Australia has been an early adopter every step of the way. This is undoubtedly one reason that the Daffodil Centre ‒ a joint venture between Cancer Council NSW (New South Wales) and the University of Sydney, which specialises in cancer control and policy – was given the lead role in developing the global elimination strategy for the WHO.
“Australia is very well positioned to eliminate cervical cancer and, in fact, is projected to do so within the next few years”
It is also why Australia can offer a great model for other countries to follow, as the impact of its control efforts are already becoming apparent, even though the full benefits are not expected to become apparent for many years yet. “Australia is very well positioned to eliminate cervical cancer and, in fact, is projected to do so within the next few years, between 2028 and 2035,” says Canfell.
HPV vaccination
The first vaccine against cancer-causing strains of HPV was approved by the US regulators in 2006. In 2007 Australia became the first country in the world to roll out a national HPV vaccination programme for girls, and in 2013, this programme was extended to include boys. In its initial phase, the programme – in addition to school-based vaccination for girls aged 12‒13 years – included a community-based ‘catch-up phase’ for women up to the age of 26 years, which ended in 2009.
The maximum impact of a prophylactic vaccine – administered to young teenagers, to protect against a cancer that peaks in older adults – is going to take some time to reach. However, data from Australia already show that, 10 years after introduction of the HPV vaccination programme, high-grade cervical disease declined substantially among vaccine-eligible women.
HPV screening
Protection against cervical cancer, particularly for women who were not vaccinated in their youth, relies on the effective functioning of the country’s population-based cervical screening programme that was first established back in 1991. “The missing piece is making sure women are participating at a higher level in cervical screening, that’s the lever that can really promote more rapid reductions in cervical cancer rates,” says Canfell.
However, stark inequalities are seen both in access to screening and in the current burden of cervical cancer. As Canfell points out, it’s a global problem. “The burden of disease is heavily concentrated and very inequitable in low- and middle-income countries. Even within high-income countries, there are many inequities in terms of which women are not participating as regularly – or at all – in cervical screening and who are thus at higher risk of cervical cancer.” In Australia, she says, inequities in the burden of cervical cancer are particularly marked in the Aboriginal and Torres Strait Islander women: incidence and mortality rates between 2011 and 2015 were two and four times higher, respectively, when compared with rates among non-Indigenous women.
One reason for this disparity, says Canfell, is that screening services have not been accessible to some groups to the same extent as they are accessible to other groups. “Historically, we had major difficulties in reaching all groups with cervical screening using the Pap smear, which needs relatively frequent screening to be effective. Pap smear-based screening hasn’t well-served certain cultural groups, and it hasn’t necessarily served gender- and sexually diverse people. There has been this huge barrier for access to a potentially life-saving screening test.”
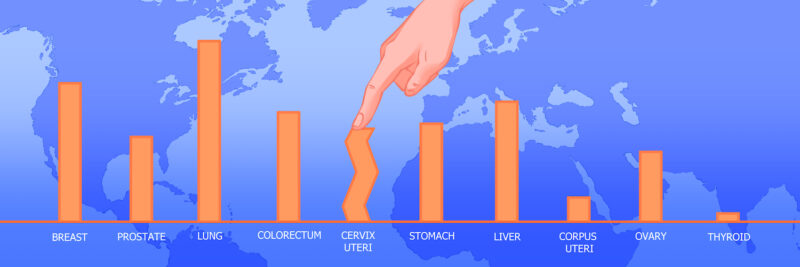
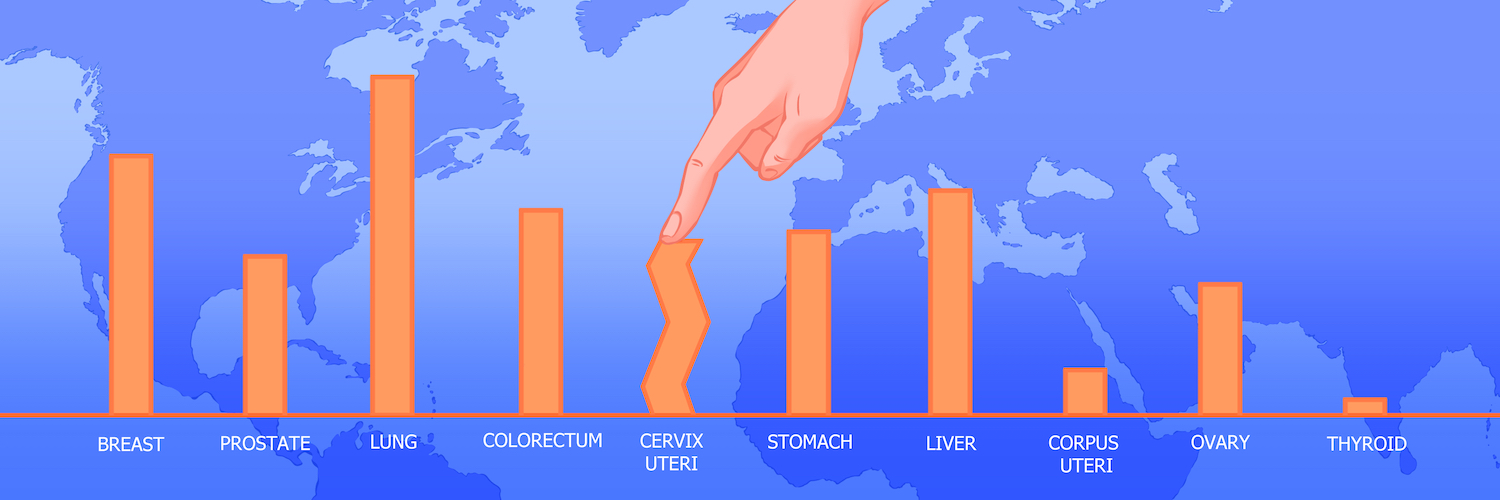
Global disparities in the prevalence of cervical cancer

The burden of cervical cancer is heavily concentrated in low- and middle-income countries.
Data source: GLOBOCAN 2020, Graphic production: IARC, World Health Organization © International Agency for Research on Cancer 2022.
HPV-based screening
Screening may become more accessible with the introduction of HPV tests, which test for presence of the virus in cervical cells, rather than looking for precancerous changes that might result from such chronic infection. In 2017, Australia switched its screening programme: instead of the traditional Pap smear test it now uses nucleic acid testing for HPV in a five-yearly interval, followed by liquid-based cytology testing for triaging HPV-positive samples.
“You could set up the test to essentially run while the woman is visiting the healthcare facility and get an instant result”
The HPV test improves screening access in two ways: First, unlike with Pap smears, HPV testing allows people to take their own sample. “In the Australian programme, a universal option for self-collection is being rolled out in mid-2022. Everyone due for cervical screening will be able to decide whether they want a practitioner to take the sample, or can take their own sample under the supervision of a practitioner, but in private.” As the test can be delivered in a different way, new models of delivery are being co-designed to improve access for traditionally underserved groups. “For particular cultural groups, there will be opportunities for communities to be involved in thinking about how best this would work, and what new models this will open up that will actually help address the accessibility issues.”
As an additional benefit, HPV tests are available as point-of-care tests and, bolstered by laboratory infrastructure built up for Covid testing, could deliver almost instantaneous results. “In the right environment, you could set up the test to essentially run while the woman is visiting the healthcare facility and get an instant result. So you can manage that result quickly, which is much more efficient.” Depending on the setting, self-collection and point-of-care testing can be combined or used separately to improve available options. “This allows a country, a population or a community to really think: What’s going to work for us, and how can we use these possibilities in a way that hasn’t been open to us before?”
Strategies for Europe
In Europe, one of the key groups asking those sorts of questions is the HPV Action Network. Established in 2019, following that year’s European Cancer Summit, the Network brings together stakeholders and organisations under the auspices of the European Cancer Organisation. It has issued four calls to action aimed at reducing the burden of HPV-related cancers and setting the WHO Europe region on track for eliminating them altogether. With some exceptions, the task ‒ and the potential benefits ‒ will be greater in central and eastern European countries, where incidence rates are on average 16 per 100,000 women, compared with western Europe, where the average is less than 7 cases per 100,000.
The Action Network’s four recommendations include: HPV vaccination for all teenagers, regardless of gender; organised population-based screening programmes using HPV tests; best-practice cancer treatment; and increased awareness and education about HPV. “We deliberately set out to present the European Union with a plan for eliminating a range of cancers,” says Daniel Kelly, co-chair of the HPV Action Network. “We set the date [for elimination] as 2030. We felt that if we set a target, we have to make people think and challenge individual countries and health systems to consider where they are in the journey.”
HPV vaccination strategies and implementation
The first call to action, on gender-neutral HPV vaccination, has been included in Europe’s Beating Cancer Plan – a big success, says Peter Baker, campaigns director for the HPV Action Network He believes gender-neutral vaccination is crucial to eliminating cervical cancer from the globe, and regrets that it is not elaborated upon in the WHO’s strategy. “If our aim is to eliminate HPV caused cancers, we have to vaccinate everybody. If the aim is to eliminate cervical cancer specifically, that will happen faster if both girls and boys are vaccinated, because boys are the main vector,” Baker explains. “If we have a girls-only vaccination programme, we are leaving men who have sex with men exposed to HPV infection. That’s an important issue that we shouldn’t overlook.”
“If our aim is to eliminate HPV-caused cancers, we have to vaccinate everybody”
Baker does see the argument for prioritising girls, e.g. in case of vaccine shortages. However, he believes that ambitions should be set beyond. “There is a logic to [prioritising]. But that’s not what should happen – we should be aiming for long term and be open to the vaccination of everybody… WHO’s position is that the priority, at this stage, should be to vaccinate girls. They don’t have a perspective on gender-neutral vaccination apart from saying ‘we’re not at that point yet’. I think it should be a more aspirational approach than that, we should be thinking about how we can move now to vaccinate everybody.”
The Network’s current priority is to see the commitments of Europe’s Beating Cancer Plan implemented across the European Union, and more broadly across the WHO Europe region. This will involve addressing the various issues behind vaccine hesitancy, says Baker. “The fact that HPV is a sexually transmitted infection gets misinterpreted into a belief that if young people are vaccinated, they will change their sexual behaviour. So there can be cultural or religious concerns that touch on this. But we know there’s no evidence that giving people the HPV vaccine leads to any changes in sexual behaviour.”
For Kelly, a successful campaign needs to show parents that protection before a sexual debut is better than vaccinating at a later point, and should convey a positive message. “The countries that have done it well respect young people’s decisions about choosing a vaccine for a positive reason, rather than it being imposed on them by the adult community,” he says.
Screening strategies
For cervical cancer screening, the HPV Action Network supports the introduction of HPV testing, already in use in Australia and in some European countries. “We need to push for the best,” says Kelly, “But if in the meantime we can get cytology-based screening, that’s better than nothing. Again, we need to make sure that people are aware that they can access screening.” Screening should also convey a positive message, rather than being associated with the idea of suddenly being confronted with a cancer diagnosis.
“The challenge will be how to bring the plan to life, especially in those countries that we see as currently behind the schedule”
In the face of stark inequalities across Europe, reaching the goal of eliminating cervical cancer requires working together and implementing the recommendations set out in Europe’s Beating Cancer Plan, says Kelly. “The challenge will be how to bring the plan to life, especially in those countries that we see as currently behind the schedule. The plan provides a fantastic target, but we need to provide those countries with a roadmap to learn from each other and provide this support through the Network.”
An EU Joint Action on HPV vaccination was announced in February 2022, as a new Beating Cancer Plan action. This will support member states to “increase public understanding and awareness of HPV and promote vaccination uptake”.
Mapping Cervical Cancer Prevention policy across Europe
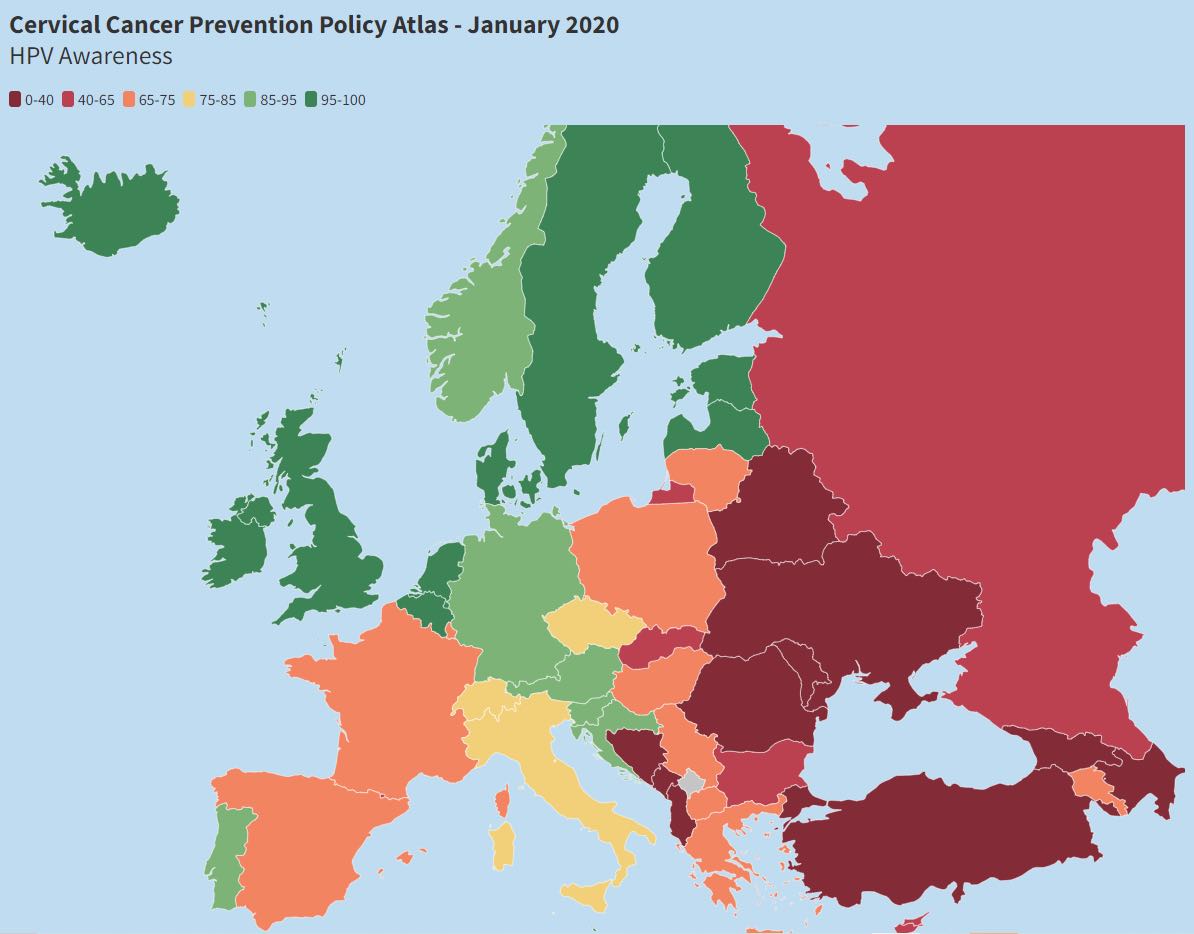
Progress across Europe in actions to prevent cervical cancer is mapped in an online Cervical Cancer Prevention Policy Atlas, published by the European Parliament Forum for Sexual and Reproductive Rights. This map shows levels of HPV awareness. Access to screening, vaccination and information, and the adoption and implementation of national policy on cervical cancer prevention are also mapped. Details on each indicator by country are provided, together with an infographic showing overall score and ranking for each country, on an information sheet published on the same website.
Source: Screenshot from the website of the European Parliament Forum for Sexual and Reproductive Rights, captured 29 March 2022.
The Slovenian model
One European country that achieved an impressive turnaround in tackling cervical cancer is Slovenia. In the 1960s, Slovenia had been one of the first countries in Europe to set up a high-quality cancer registry. This meant that public health authorities were alerted when incidence rates started rising in the 1990s. At that time, screening was available but only on an ‘opportunistic’ basis ‒ it relied on patients or doctors making the request, rather than an organised programme with guidelines for instance on who should be screened, how, and how often.
Urška Ivanuš, public health specialist at the Institute of Oncology Ljubljana, Slovenia, and Head of the national cervical cancer screening programme ZORA recalls that a similar rise in incidence was being seen in other eastern European countries in the 1990s, where there was no screening or only opportunistic screening, but that no such increase was noted in Nordic countries, where organised cervical cancer screening programmes were up and running. It was a wake-up call for Slovenia, she says. “We realised: something is not working, we have to organise our screening. We started a first pilot in 1998, which became a national programme in 2003.”
Screening strategies
Ivanuš recalls the many challenges they encountered setting up the organised screening programme, one of which was changes to the parameters of screening. While opportunistic screening was available to everyone, the new programme limited screening to women aged 20 to 64. “Typically, under the old model, the same women – usually those more aware and more educated – would come too often, as the usual recommendation was to do a Pap test every year. And we then prolonged the screening interval to three years.”
“We have this shared vision now that Slovenia will be one of the first European countries to eliminate cervical cancer”
The change to an organised screening programme yielded results. “We put a lot of effort in to increase the coverage as much as possible, we implemented standards and professional guidelines, and we established a screening registry – so we know exactly which women are screened correctly. Fairly soon, we found that we managed to lower the referral rate, so we screened more women but had fewer to refer further. We were detecting more pre-cancers and were lowering the incidence of cervical cancer. Slowly, trust increased. We have this shared vision now that Slovenia will be one of the first European countries to eliminate cervical cancer.”
Slovenia is planning to switch from cytology-based screening to HPV tests in the coming years, and to further prolong the screening interval. One reason is to avoid a return to opportunistic screening, this time using HPV tests, e.g. offered over-the-counter in pharmacies as self-sampling tests. “If we do not offer it in an organised way, I’m sure that it will come in as an opportunistic screening. Opportunistic is not an option, because it is always worse than organised screening.”
As she points out, a particular problem with opportunistic HPV screening is the high incidence of HPV infection in young women, where the benefits are not worth the downsides, which is why HPV testing is not currently recommended for young women who are not vaccinated.
“Every fourth woman aged 20 to 24 in Slovenia is positive for an HPV infection with a viral type known to cause cancer. We can’t treat the HPV infection: it will clear without treatment, or it will expose them to further disease. They can pay for the test, but then they become a burden on the health system – and we can’t actually offer them anything, at this point. The benefits of testing do not balance the negatives.”
Instead, the timing of HPV tests should be defined for maximum effect ‒ the evidence suggests that HPV screening has greater benefits than cytology over the age of 30. “This is, of course, different in HPV-vaccinated cohorts, because their HPV prevalence is much lower, so this might change when the vaccinated cohort reaches this age. But we try to do as little screening as possible to achieve the best results possible, and still catch serious disease early enough.”
Vaccination strategies
HPV vaccinations could also contribute to reducing inequality in the incidence of cervical cancer. “This is a difference that can be lowered with HPV vaccinations – or even enlarged.” Slovenia implemented HPV vaccination in 2009, and reached a vaccination coverage of around 50% for several years, which rose to 60% in the year prior to the Covid pandemic. In September 2021, the vaccination programme was extended to cover boys in a school-based vaccination programme.
“Childhood vaccinations are compulsory in Slovenia… We are not used to campaigning for vaccinations, but we will have to”
So far, no data is available for how vaccine uptake changed after the pandemic. According to Ivanuš, HPV vaccination is a high political priority in Slovenia, but it also encounters a rather specific challenge: “All the childhood vaccinations are compulsory in Slovenia, so we did not put in much effort in the past to increase vaccine health literacy,” she explains. HPV vaccination is not compulsory, so the communication has to be adjusted. “We are not used to discussing vaccinations and campaign for vaccinations, but we will have to. Also, when and if the evidence will be strong that HPV vaccination is safe and effective also in younger ages, with other childhood vaccines, it could make logistics as well as communication easier.” Ivanuš sees an opportunity in addressing the whole cervical cancer prevention age spectrum, as “the parents are the mothers – who are also the candidates for cervical cancer screening”.
Lessons from Slovenia
What lessons can be learned from Slovenia? A lack of organised screening is what sets eastern European countries with higher incidence apart from Slovenia, says Ivanuš. She points out that HPV testing offers a huge opportunity for these countries. “It’s also an art how to integrate such a complex organised programme into the healthcare system that typically has a lot of problems, at least in some countries.”
Slovenia is participating in EU projects to transfer its experience with organised screening programmes – also for colorectal and breast cancers – to other eastern European countries, such as EU-TOPIA-EAST. “With the Europe’s Beating Cancer Plan and the WHO goal, there are a lot of new opportunities for countries which were not successful in the implementation of HPV vaccinations or screening. But the time to act is now,” she stresses. She acknowledges that no ‘cookbook’ exists for implementing successful screening programmes. “It’s not easy. Even though we have the knowledge, it’s still difficult to transfer this knowledge into practice. For HPV vaccination, it is much, much simpler – but in each community, you have to approach it in the way that the community will accept it.”
Illustration by Alessandra Superina