Interview with Christian Rolfo, MD, PhD, MBA, Dr.h.c. director of the Thoracic Medical Oncology and the Early Clinical Trials and Experimental Therapeutics Research Program investigator at the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center.
Q. Your article in Nature Reviews Clinical Oncology (vol 17, September 2020, page 523) reflects on the integrated genomic strategy proposed by Chabon et al. to detect early stage lung cancer using a cfDNA machine-learning platform. Which are the main limits of this approach?
A. The results of the study of Chabon et al. are very promising and suggest that cfDNA analysis, using the Lung-CLiP algorithm, might provide, in the next future, a valuable tool for early lung cancer detection. A good point of this study is the auto-validation. However, this study has some important limitations. For instance, the majority of patients included in the study were smokers and had incidentally diagnosed cancers, leaving unresolved the performance of this test in the LDCT-screened population and in never-smokers. Moreover, the sample size was too small and large- scale reproducibility of this test should be explored further.
Q. Do you really believe that liquid biopsy approaches could revolutionise lung cancer screening?
A. Liquid biopsy can potentially revolutionise lung cancer screening due to its minimally invasive approach and the possibility to repeat over time the tests. If the technical limitations against we are struggling at the moment would be overcame in the next future, circulating tumour DNA (ctDNA) might represent a game changer in early-stage lung cancer saving thousands of lives. However, it is unlikely that liquid biopsy can reach sufficient sensitivity and specificity for cancer detection without integrating data derived from these analyses with other methodologies, such as low-dose CT (LDCT) scan. The use of liquid biopsy in this setting will be likely not a substitute for currently validated screening approaches, but rather might represent a pre-screening tool for reducing the costs and socio-economic burden of LDCT programs and/or integrate data from these screening methodologies in order to reduce false positive results and minimise the risks of unnecessary invasive procedures.
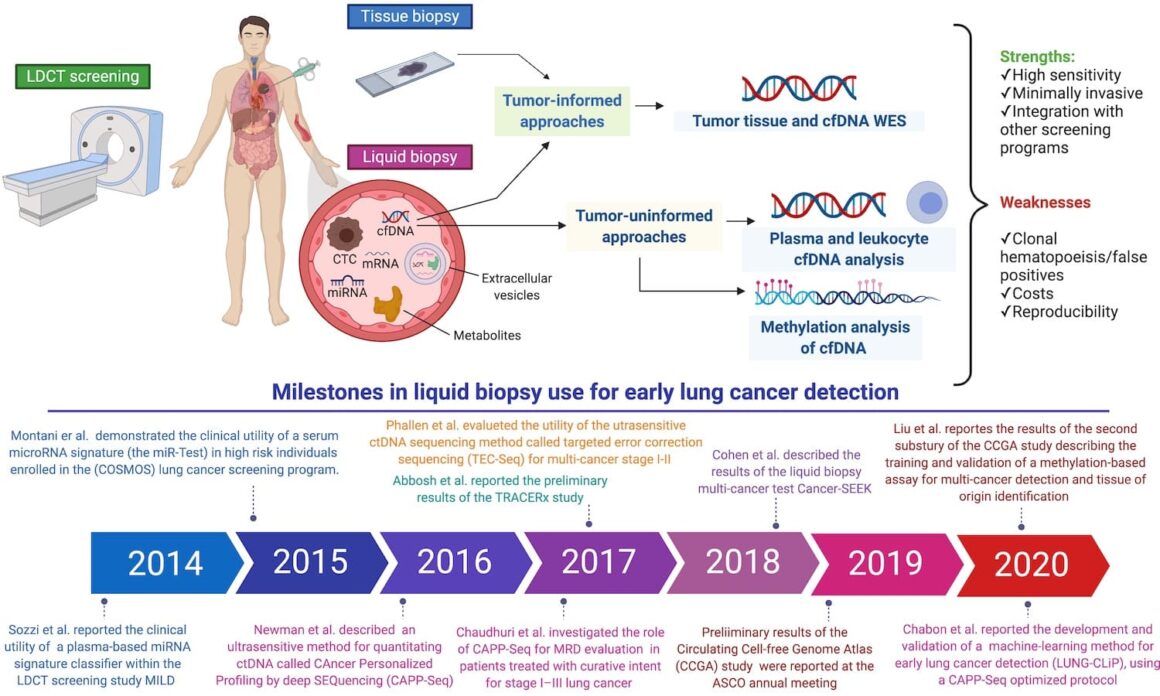
Q. Do you still see a future for low-dose CT scan screening programmes?
A. In my opinion, at least in the near future, liquid biopsy could not replace definitely LDCT screening, but rather might represent a complementary tool either in the pre-screening (Which patient should be screened with LDCT scan) or post-screening (Which suspect nodule require further invasive procedures, such as bronchoscopy or CT-guided biopsy) setting.
Q. Which is the essential message of your paper and which illustration expresses it better?
A. In our editorial, we expressed our interest and enthusiasm for the great advances made in the last few years with the use of ctDNA for early lung cancer detection. However, it is still a long way to go before clinical implementation. Several technical and biological limitations hamper the adoption of liquid biopsy for this purpose at the moment and further studies are required. Notwithstanding these limitations, the study of Chabon and colleagues represent a major step forward in the field and pushes ctDNA analysis for early lung cancer detection into a novel dimension. An integrative approach with conventional LDCT screening and, likely, with other components of the large liquid biopsy family might represent the key for success.
About the author
Christian Rolfo, MD, PhD, MBA, Dr.h.c.
Professor of Medicine
Director of Thoracic Medical Oncology Program
Director of Early Clinical Trials
Experimental Therapeutics Research Program
Marlene and Stewart Greenebaum Comprehensive Cancer Center
University of Maryland School of Medicine









