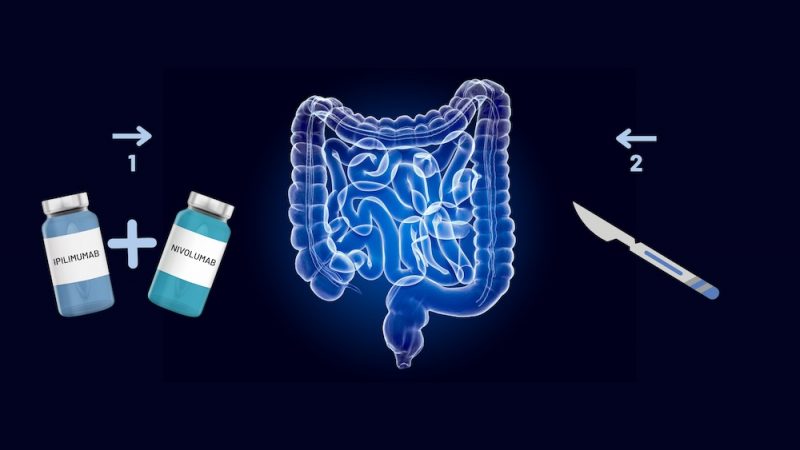
In patients with non-metastatic colon cancer who have mismatch repair deficient tumours, a short course of neoadjuvant immunotherapy (one cycle ipilimumab and two cycles nivolumab) was highly effective. The NICHE-2 trial, published in the New England Journal of Medicine, June 5, showed that the immunotherapy regimen had an acceptable safety profile, with 95% of patients achieving a major pathological response and 68% a pathological complete pathological response.
“Now, more than two years after treatment, none of the patients saw recurrence even though many had high risk tumours. These results are unprecedented. The efficacy as well as the side effects are much better compared to chemotherapy before surgery, a treatment that only works in one out of 20 patients,” says Myriam Chalabi, the first author, from the Netherlands Cancer Institute, Amsterdam.
Deficient mismatch repair (dMMR), also known as microsatellite instability, occurs when cells have impaired DNA repair mechanisms leading to the accumulation of mutations. Estimates suggest that around 10 to 15% of patients with non-metastatic colon cancer have dMMR tumours.
Recent data from the FOxTROT study supported the use of neoadjuvant chemotherapy in patients with locally advanced colon cancer on the basis of improved two-year disease control. However, only 7% of patients with dMMR tumours achieved pathological responses, suggesting that chemotherapy may be less effective for this group. In other studies, immune checkpoint blockade has been shown to significantly improve survival in patients with dMMR metastatic colorectal cancers. The rationale for giving immunotherapy prior to surgery is to familiarise the immune system with tumour variations before the tumour is removed.
The NICHE-2 trial was initiated after a pilot study, published in Nature Medicine in 2020, showed that patients with non-metastatic dMMR colon cancer achieved a 100% rate of pathological response and a 60% rate of pathological complete response when treated with dual checkpoint blockade.
In the phase 2 investigator-initiated NICHE-2 study, between July 2017 and July 2022, 115 patients, with non-metastatic, locally advanced, previously untreated dMMR colon cancers, were treated with neoadjuvant nivolumab plus ipilimumab. Patients received two doses of nivolumab at a dose of 3mg per kilogram of body weight, with the first dose administered on day 1 and the second on day 15, and one dose of ipilimumab at a dose of 1mg per kilogram on day1. Surgery was scheduled to be performed within six weeks after study enrolment. An experienced gastrointestinal pathologist assessed the percentage of residual viable tumours in resection biopsy specimens sampled during surgery.
The team decided against performing a randomised study. “In the case of non-metastatic dMMR colon cancers, the substantial gap between response to neoadjuvant chemotherapy and response to neoadjuvant immunotherapy, in our opinion, renders a randomised trial comparing these treatments unethical,” write the authors.
Results show that, of 115 enrolled patients, 113 underwent timely surgery, with only two patients having surgery delayed by more than two weeks.
Among 111 patients included in the efficacy analysis, a pathological response (defined as less than 10% residual viable tumour) was observed in 95% (n=105) and a pathological complete response (defined as 0% residual viable tumour) was observed in 68% (n=75).
A pathological complete response was observed in 79% of patients with Lynch syndrome vs 61% without Lynch syndrome.
With a medium follow-up of 26 months (range 9–65 months) no patients showed disease recurrence.
“The high proportion of patients with a pathological response observed after only 4 weeks of treatment in our study, together with the safety profile, may provide sufficient justification to provide immunotherapy to patients with radiographically assessed high-risk disease, especially if 3-year disease-free survival data from this study are positive,” write the authors.
In an accompanying editorial Andrea Cercek, from Memorial Sloan Kettering Cancer Center, New York, writes, “Although it is exciting to see such marked responses, these results alone do not support the adoption of this approach as the standard of care for patients with early-stage dMMR colon cancer. It is important to recognise that surgical resection alone is curative in the majority of stage II dMMR colon cancers and that adjuvant therapy applies predominantly to stage III disease. Staging of dMMR colon cancer is especially challenging, given that tumors with microsatellite instability are often associated with falsely positive enlarged lymph nodes owing to immune infiltration. Thus, a substantial proportion of patients with dMMR colon cancer would not need any treatment beyond surgical resection.”