Ni Miikana is a Native American expression meaning “my path”. My path, my journey in science is not about me, it is about the institutions that have supported us and the many mentors and mentees who made this talk possible.
250 years ago, a French philosopher, Voltaire, said: “Doctors pour drugs they know little about, to cure diseases they know less about, into patients they know nothing about.” Much progress has been made since then and many cancers are now under control or cured. However, much work still remains to be done.
Up until recent times, the three pillars of cancer treatment were surgery, radiation, and drugs. The concept of chemotherapy was born in Italy. In 1943, a US Army doctor, Stewart Alexander, noticed that after the German bombing of Bari, some people died with shrunken lymph nodes. He also suspected that the cause was nitrogen mustard gas, a chemical warfare agent. Goodman and Gillman of Yale University also treated their lymph node cancer patients with nitrogen mustard. Subsequently, many chemotherapy drugs were developed. They have proven to be very useful. Yet, their use is limited by their toxicity.
In the 21st century, very few of these anticancer drugs are being developed. Now, if we know what causes cancer, we can design drugs to target it. It is like a horse pulling a cart. For the last 25 years scientists have discovered what horses cause cancer, the so-called oncogenes, and scientists and pharmaceutical companies made drugs to target them. Many of these drugs have proven to be very useful. However, their number is limited. Millions of patient genomes have now been sequenced, and we know there are few targets left unexplored.
“In the post chemo and post anti-oncogenes era, the new target of oncology is to stage-manage fire and water”
So, what is the future of cancer treatment? “It is with our passions as it is with fire and water, they are good servants, but bad masters,” as the ancient Greek fabulist and storyteller Aesop once said. In the post chemo and post anti-oncogenes era, the new target of oncology is to stage-manage fire and water. When fire is on and water is not on, over a period of years, cancer develops. It goes down three paths. First, the metabolism of the cancer cells changes; second, they cannot efficiently repair the two billion base pairs of DNA, and lastly they evade the immune system that protects us.
Targeting cancer metabolism
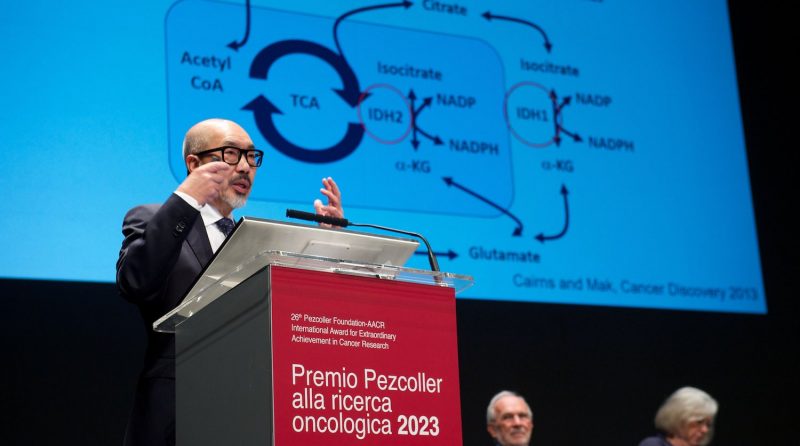
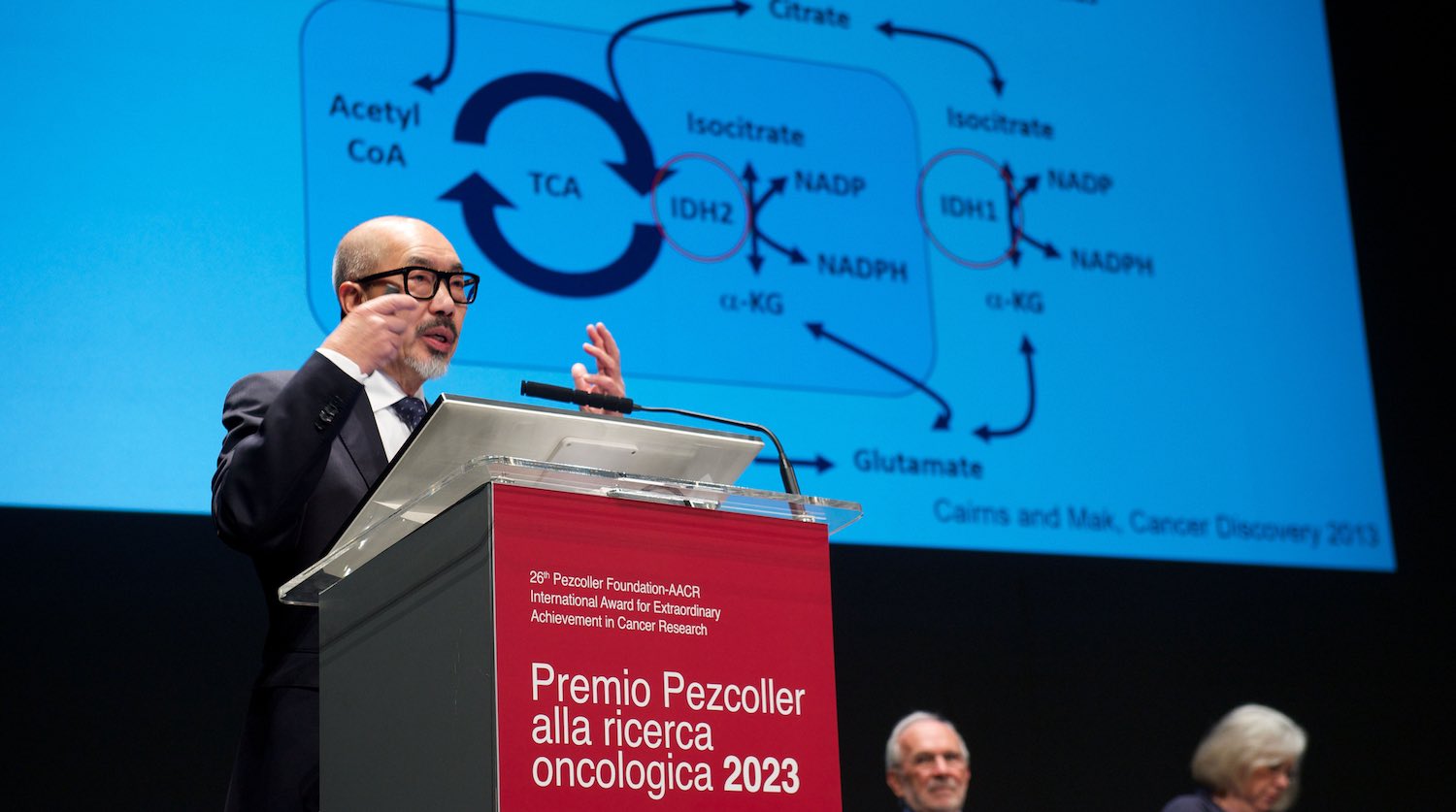
Metabolism is very complicated, with 2,500 genes involved in regulating glucose, lipids, and amino acids synthesis. These genes also control energy production and consumption. One of the targets of metabolic adaptation is the enzyme isocitrate dehydrogenase (IDH). Interestingly, this is mutated in many tumours. Mutated IDH was first discovered by Bert Vogelstein’s lab in brain tumours. Our lab, together with the French group of Phillipe Gaulard, found it in lymphomas. We have conducted extensive studies of several types of cancer, including acute myeloid leukaemia and T-cell lymphoma. This metabolic mutation appears to affect metabolism modulation and epigenetics as well as DNA repair.
Recently, two French researchers, Julie Leca and François Lemonnier, working with us, demonstrated that metabolic mutations in T-cell lymphoma also modify the tumour microenvironment and cooperate with B cells to drive both T and B cells cancer formation: it is practically like shaking hands with the devil.
With Lew Cantley and Craig Thompson, we founded Agios Pharmaceuticals in 2008. Thanks to the hard work of many company members, we developed three drugs against these targets. Two of them have been approved, for leukaemia and cholangiocarcinoma. One of the most difficult cancers to treat is brain tumour which also has this mutation. Agios – now the French company Servier – recently announced that the third drug, vorasidenib, has reached the endpoint of a phase III trial in IDH-mutant low-grade glioma.
Tuning our immune system
Confucius once said: “We all have two lives: The second begins when we realise we only have one.” Cancer has reminded many of us how true this concept is. Even modern medical scientists have two lives: The second begins when we realise that our immune system, which is supposed to protect us from viruses and bacteria, is also the orchestra to most of life’s symphonies.
Our immune system developed because we have to fight viruses, bacteria, parasites. It is a system that fights foreign entities, but sometimes it fights itself. You get autoimmune diseases, you get neurodegenerative diseases… and sometimes you get cancer.
Discovering the T cell receptor
What is the fundamental sensor that allows immune cells to kill cancer cells and virus-infected cells, and yet not damage our own organs? The key, the main antenna of the immune system, is the T cell receptor. It detects the virus, the cancer cell, it recognises the human leucocyte antigen. It serves to identify a pathogen to kill, such as Covid 19, without affecting normal organs.
Until the 1980s, the true identity and structure of this immune sensor eluded discovery. The T cell receptor was finally identified by Yusuke Yanagi, a Japanese post-doc in our lab, who used an unconventional approach to clone T cell receptor genes for the first time. This molecular technique is based on looking for the transcriptional differences between T and B cells. The same result was also independently achieved by Mark Davis at Stanford. The two works were published together in 1984.
Discovering the immune system brake lever
The best way to study the complex physiological functions of each gene is to generate genetic models in mice. Paul Waterhouse, a Canadian doctor in our lab, found out that one of the genes, CTLA-4, is the brake on T cell function, the T cell receptor is the key, and CD28 is the accelerator. However, I never thought this was the beginning of treating cancer by immunotherapy. I continued to curse the darkness, not finding how immune cells could be helped to kill cancer cells.
“Paul Waterhouse, in our lab, found out that CTLA-4 is a brake… James Allison got a Nobel prize for developing an antibody to block it”
James Allison, who won the Pezcoller/AACR prize a few years ago, and went on to win a Nobel Prize, showed better insight. He lit the candle, and developed an antibody to block the CTLA-4 brake. This approach stimulates the immune system to attack the cancer cells. It was the beginning of immunotherapy, the fourth pillar of cancer treatment. Many cancers have shown a very strong response, and many patients can now be considered ‘long term survivors’. Tasku Honjo, in Japan, developed the widely used anti-PD1 immune checkpoint, and was also awarded the Nobel Prize, together with Allison.
Interleukin-7 to re-energise tired T cells
The tumour microenvironment may be the main reason for the success or failure of immunotherapy. It is a complex ecosystem comprising various components, including stromal cells, macrophages, and several others, that can influence the activities of anti-tumour responses. Intensive studies are conducted by thousands of laboratories around the world. One property of killer T cells is that they can become depleted or exhausted. In our laboratories we discovered that interleukin-7 can give them the energy to fight again. Giving a mouse interleukin-7, allows the killer T cells to fight for another hundred days before the mouse dies. This drug is now in human clinical trials.

Breaking through cancer cell defences
Killer T cells move into the tumour area to kill the tumour, but cancer cells defend themselves. They build a wall and trap the T cells in blood vessels, blocking them from entering. We need to act as in the legend of Troy. The Trojans built a wall to keep their Greek attackers out. But the Greeks hid inside a wooden horse to gain entry inside the walls. But what is this wall in our body? And what can the immunological Trojan horse be? The wall turned out to be macrophages and blood vessels, preventing T cells from getting in.
“T cells bring their own key to open the door and exit the blood vessels to kill tumours”
Unexpectedly, part of the Trojan horse comes from a message from the brain. The brain makes a neurotransmitter, norepinephrine, to activate killer T cells. The T cells then produce another neurotransmitter, acetylcholine. Kevin Tracy in New York and our lab discovered, more than 10 years ago, that T cells can create neurotransmitters. An American post-doc in my lab, Maureen Cox, made a crucial experiment in mice. She has shown that when killer T cells fail to produce the neurotransmitter acetylcholine, they cannot clear a viral infection. In other words, T cells bring their own key to open the door and exit the blood vessels to kill tumours.
Most interestingly, Geoffrey Watson, working under the guidance of Eric Chen and Lillian Siu in Toronto, demonstrated that there is a relationship between the level of choline – a precursor to acetylcholine – and response to immunotherapy. Patients with high choline levels perform better than patients with low blood choline levels when treated with immunotherapy.
Where next? Modifying T cell receptors for solid tumours
Finally, I would like to go back to where it all began: the T cell receptor, the key to identifying the cancer cell. T cells are like superheroes from movies, like James Bond and Jason Bourne. They can enter a tumour, find, and kill the enemy. We need to find the James Bonds and the Jason Bournes and treat cancer with them.

This has already been done. Carl June and Michel Sadelain took a T cell receptor and hooked it up to an antibody to create a chimeric antigen receptor T cell – known as CAR T cell. CAR T cells have been successful in treating leukaemia and lymphomas, and are FDA approved.
But, what about pancreatic, breast, and prostate cancer, etc.? For solid tumours like these we would need T cells engineered to express modified T cell receptors, known as TCR-Ts. The challenge for TCR-T is to identify the specific T cell receptor for cancer antigens and to define hereditary human leukocyte antigens.
Tremendous progress is being made in the field of TCR-T for solid tumours. TCR-T against the testicular cancer antigen NYES-01 is effective for some sarcomas. And TCR-T specific for human papilloma virus E7 viral antigen is also active against some cancers of the cervix, rectum, and head and neck. Using TCR-T for the treatment for uveal melanoma, a highly challenging form of cancer to treat, has been approved by the US regulators, the FDA.
Naoto Hirano’s laboratory in Toronto has developed a highly sensitive platform to identify T cell receptors against many tumour antigens covering many types of cancer in more than 90% of European, Asian, and African populations. We are thrilled to collaborate with him on this ambitious project.
In conclusion, it is important to remember that we are just one-winged angels, as a famous Italian writer, Luciano De Crescenzo, said. We can only fly together. Over the past 30 years, 150 doctors from all parts of the world have participated in our laboratory’s research. There is an African saying: “If you want to go fast, go alone; if you want to go far, go together.” A big thank you goes to the institutions that have supported me for over four decades, and all the post-docs, students, technicians, and collaborators for their working together to go far. Finally, thanks to AACR and the Pezcoller for their kind recognition.
This text was published with the kind permission of Tak Mak.
The speech was delivered by Tak Mak at an award ceremony held by the Pezcoller Foundation in Trento. He also presented at the 2023 Annual meeting of the American Association for Cancer Research (AACR), in Orlando, Florida, where he received the Pezcoller Foundation‒AACR International Award for Extraordinary Achievement in Cancer Research. It is published as redacted by Adriana Albini.
The Pezcoller Foundation‒AACR International Award for Extraordinary Achievement in Cancer Research was established in 1997 to recognise a scientist of international renown who has made a major scientific discovery in basic cancer research or who has made significant contributions to translational cancer research.
Tak Mak is a senior scientist at the Princess Margaret Cancer Centre, University Health Network, as well as a university professor in the departments of medical biophysics and immunology at the Temerty Faculty of Medicine, both at the University of Toronto. He is also a professor in the department of pathology at the University of Hong Kong.
The award was granted in recognition of his role “leading the group that cloned the human T cell receptor beta chain, a key component of the immune response, which has helped stimulate a remarkable series of advances in cancer immunology research”. It also recognised “his enormous contributions to cancer biology, highlighted by his generation of genetically modified mouse models, which have allowed for in-depth investigations into cancer initiation and progression.”