Blocking the activity of an ion channel in a mouse model of cancer triggered metastasis independent of tumour formation. The study, published in Nature Genetics, 29th September, found that the process was not just restricted to mice with cancer; when NALCN was removed from mice without cancer it caused healthy cells to leave their original tissue and travel around the body.
“Not only have we identified one of the elusive drivers of metastasis, but we have also turned a commonly held understanding of this on its head, showing how cancer hijacks processes in healthy cells for its own gains. If validated through further research, this could have far-reaching implications for how we prevent cancer from spreading and allow us to manipulate this process to repair damaged organs,” says Richard Gilbertson, Group Leader for the study and Director of the Cancer Research UK Cambridge Centre.
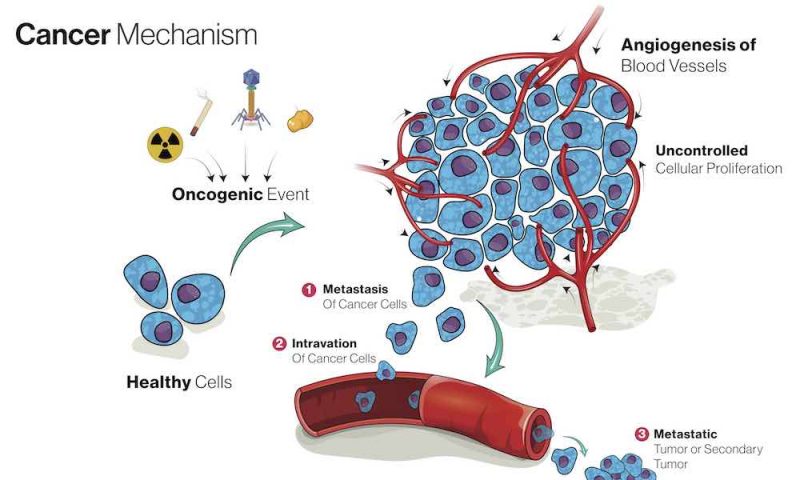
The most common cause of cancer death is metastasis, the process by which cancer cells spread from the primary tumour to other organs, which ultimately leads to their loss of function. Blocking the process of metastasis could markedly improve the survival of patients with cancer, but how the process is triggered within the complex cascade of tumorigenesis has been unclear. “Because metastasis involves so many different processes, it has been hard to uncouple the processes involved in cells actually leaving the primary tumour from characteristics of the primary tumour itself,” says Eric Rahrmann, the lead researcher.
In the paper the investigators identify a single ion channel, sodium leak channel non-selective protein (NALCN), as a key regulator of epithelial cell trafficking to distant tissues. NALCN is known to be responsible for the background sodium leak conductance maintaining the resting membrane potential. It is known to regulate key functions in excitable tissues, for example respiration and circadian rhythms, and gain-of-function mutations have been associated with neurological disorders.
However, little is known about the role of NALCN in non-excitable tissues. The team first focussed on NALCN following experiments comparing gene expression profiles of normal and malignant gastric stem cells to identify the earliest changes in tumour initiation. They identified a selective silencing of ion channels and solute carriers in the malignant stem population. One of these genes, Nalcn, encodes the ion channel responsible for the resting membrane potential of cells, and was the only gene significantly mutated in human gastric cancer. Among 10,022 human cancers within The Cancer Genome Atlas, they found that NALCN loss of function mutations were shown to be enriched in gastric, colorectal, lung, prostate and head and neck cancer.
First, the team showed that deletion of NALCN from gastric, intestinal or pancreatic adenocarcinomas in mice did not alter tumour incidence, but increased the number of circulating tumour cells (CTCs) and metastases.
Next, they took their control mice (where NALCN had not been deleted) and treated them with gadolinium – a NALCN channel blocker – and showed that animals treated with gadolinium had significant increases in CTCs and metastasis. “Not only could we do this genetically but also pharmacologically recapitulated these findings as well,” says Rahrmann.
Finally, deletion of NALCN from mice that lacked oncogenic mutations, and never developed cancer, caused shedding of epithelial cells into the blood at levels equivalent to those seen in tumour-bearing animals. These cells trafficked to distant organs to form normal structures including lung epithelium and kidney glomeruli and tubules.
“We have uncoupled the process of metastasis from cancer. Metastasis in itself appears to be a normal process, with NALCN regulating this process and also mobilising endogenous epithelial stem cell pools for tissue regeneration,” says Rahrmann.
In the future, manipulation of NALCN could function as a promising new approach to block metastasis. For example, drugs capable of reopening the NALCN channel might offer effective antimetastatic therapies. “When a person is diagnosed with a primary tumour you could prescribe a treatment that reduces metastasis right away to prevent the shedding of tumour cells during surgery,” suggests Rahrmann.
The group now plan further studies, including looking more ‘mechanistically’ to explore what regulates the expression of NALCN, looking at the metastatic potential of specific NALCN mutations, and extrapolating the work to other types of cancer. “Since NALCN is not the only ion channel that regulates resting potential, we are looking more globally to see whether other types of channels could also be implicated in a similar process,” says Rahrmann. “We are also looking at tissue generation to see if we can harness already existing stem cell pools within our body to leverage them to repair damaged tissue.”
One concern raised by the study that needs to be addressed, he adds, is that limited exposure to gadolinium-contrast imaging has been linked to gadolinium-induced systemic fibrosis, which in cancer patients could accelerate metastasis.