A short course of induction chemotherapy prior to chemoradiation improved survival in locally advanced cervical cancer. The study, presented in a Presidential Symposium at the ESMO 2023 Congress, abstract LBA8, found that induction chemotherapy (paclitaxel and carboplatin) prior to chemoradiation taken for just six weeks reduced the risk of death by 39% and disease progression by 35%.
“Induction chemotherapy with weekly paclitaxel and carboplatin delivered immediately before chemoradiation should be considered the new standard in locally advanced cervical cancer and is feasible across diverse healthcare settings, including low- and middle-income countries,” said Mary McCormack, the study lead from UCL Cancer Institute, London, and University College Hospital, London.
In 2020 there were 604,000 new cases of cervical cancer worldwide and 324,000 deaths, of which 90% occurred in low- and middle-income countries. Since 1999 the standard of care for locally advanced cervical cancer has been cisplatin-based concurrent chemoradiation followed by brachytherapy. However, despite such treatment, up to 30% of patients will still relapse and die from metastatic disease.
For the GCIG (Gynecologic Cancer InterGroup) Interlace study, the investigators postulated that a short course of weekly ‘dose-dense’ paclitaxel and carboplatin chemotherapy before chemoradiation might downstage local disease, lengthen the exposure to systemic treatment and improve outcomes. A single-arm feasibility study, published in the British Journal of Cancer in 2013, involving 46 patients, demonstrated a good response rate to short-course weekly induction chemotherapy delivered before standard chemoradiation.
“The rationale was that the dose-dense schedule might overcome potential resistance and, by avoiding the gap between chemotherapy and radiotherapy, you limit the potential for tumour regrowth,” McCormack told Cancerworld. The team also introduced paclitaxel, since taxanes have been shown to be highly active in gynaecological cancers.
In the phase III GCIG Interlace study, between 2012 and 2022, 500 patients with newly diagnosed squamous, adeno, or adenosquamous cervical cancer, stage range from IB1 node positive to IVA, were randomised 1:1 to the experimental arm, where they received initial induction chemotherapy followed by standard chemoradiation (n=250) or to standard chemoradiation alone (n=250). In the experimental arm, patients received induction therapy with carboplatin at area-under-the-curve 2 plus paclitaxel at 80 mg/m2 once per week for six weeks before beginning chemoradiation in week seven. In both arms, chemoradiation consisted of 40 mg/m2 of cisplatin once every week for five weeks plus external beam radiation ranging from 40 Gy to 50.4 Gy, given in 20 to 28 fractions plus brachytherapy, to give a minimum total biologically equivalent dose of 78 Gy to Point A. It was later recommended that patients receive image-guided adaptive brachytherapy. Participants, who had a median age of 46 years, were recruited from 32 centres across the UK and from Mexico, India, Italy, and Brazil.
Results showed that around 75% of enrolled participants presented with stage IIA or IIB disease, 82% showed squamous histology, and almost 60% were node negative. Adherence to induction chemotherapy was high, with more than 90% receiving at least five cycles of induction chemotherapy, and more than 90% adhered to radiotherapy in both study arms.
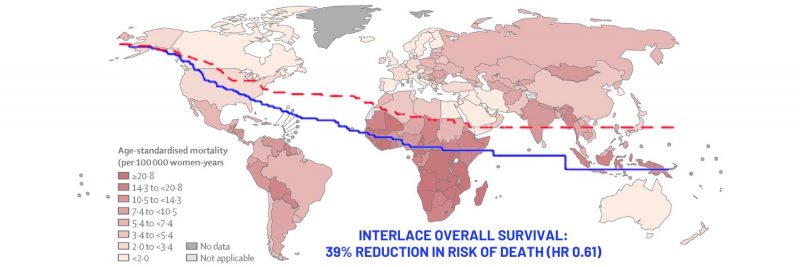
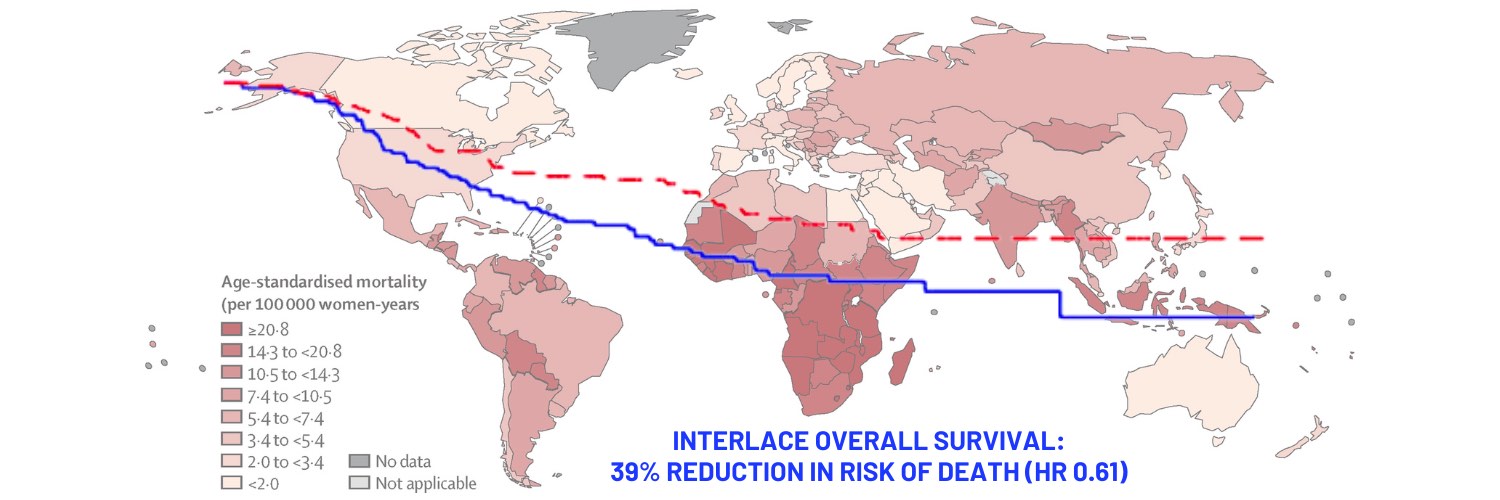
Results at five years showed that patients in the combination therapy arm had a survival rate of 80% versus 72% in the chemoradiation alone arm (HR 0.61; 95%CI 0.40–0.91, P=0.04). Additionally, progression free survival was 73% in the combination therapy group vs 64% in the chemoradiation alone arm (HR 0.65; 95%CI 0.46–0.91, P=0.013). Notably, the rate of local and pelvic relapses was 16% in both arms, but the proportion of women with distant relapses was 12% in the combination arm vs 20% in the chemoradiation alone arm. “The reduction in distant relapses suggests that the induction chemotherapy is treating micro metastatic disease,” said McCormack.
Rates of grade 3 or worse adverse events were 59% for the combination therapy group and 48% for the chemoradiation alone arm. Any grade haematologic adverse event was reported in 30% of patients in the combination arm vs 13% in the standard chemoradiation alone arm, including neutropenia (19% vs 5%), anaemia (5% vs 4%), and thrombocytopenia (5% vs 2%). “As anticipated, haematologic toxicity was greater in the experimental arm, but this did not compromise the delivery of chemotherapy,” said McCormack.
The large magnitude of benefit, added McCormack, was all the more impressive given that the duration of additional treatment was only six weeks. “Many of the other advances in frontline treatment of cervical cancer, whilst very welcome, have only been shown to reduce the chances of cancer recurring, but have not yet shown improvement in survival. The duration of treatment with the immunotherapy drugs is two years,” she said.
Next the team plan to conduct a subgroup analysis looking at outcomes according to the nodal status of patients, to understand the suitability of the approach for different risk groups. Going forward, McCormack said, it would be interesting to explore whether induction chemotherapy can be combined with immunotherapy.
The discussant, Kishnansu Tewari, from University of California, Irvine, said, “This is the first phase III trial in locally advanced cervical cancer to show a survival benefit in over two decades. Importantly this study is using drugs that are readily available and physicians taking care of these patients could consider induction chemotherapy with paclitaxel and carboplatin tomorrow morning. In those parts of the world where there may not be access to checkpoint inhibitors, patients will have this option of induction chemotherapy followed by definitive chemoradiation.”
However, whether patients with node negative stage IIA or IIB cervical cancer need induction chemotherapy was open to question. “They are probably curable with standard chemoradiation plus brachytherapy. The real risk is for stage IIIB and stage IVA patients,” he said.
Opening illustration: The map shows age-standardised mortality rates from cervical cancer in 2020. Data are from the GLOBOCAN database, collated by the International Agency for Research on Cancer and hosted by the Global Cancer Observatory
Source: Deependra Singh, Jerome Vignat, Valentina Lorenzoni, et al (2022) Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Global Health 11(2):e197–e206
© 2022 World Health Organization
Republished under a Creative Commons Attribution-Noncommercial-NoDerivs IGO (CC BY-NC-ND 3.0 IGO) licence