Around the start of the millennium, the therapeutic landscape for chronic myeloid leukaemia (CML), a rare type of blood cancer affecting the bone marrow and white blood cells, was transformed by the arrival of a revolutionary cancer drug. This was imatinib (Glivec), the first of the rationally designed, small-molecule tyrosine kinase inhibitors (TKIs) that have since proliferated for use across a wide range of cancers. The drug works by targeting BCR-ABL, the fusion gene that results from a translocation between chromosome 9 and 22 (also known as the Philadelphia chromosome), which produces an abnormal protein in the bone marrow leading to overgrowth of red and white blood cells.
Almost overnight, it transformed CML from a disease with a dismal prognosis, where only one in five patients lived more than 10 years, to a chronic condition where taking a daily pill enabled patients to achieve normal life expectancies. It also signalled a strategic change in approach to cancer therapy, towards long-term containment through inhibiting pathological signalling pathways with ‘cytostatics’, in contrast to the traditional strategic aim of killing every cancerous cell using ‘cytotoxics’.
TKIs (including first generation imatinib, second generation dasatinib, nilotinib, radotinib, and bosutinib, and third generation ponatinib) allowed CML patients to achieve a functional cure, where most people can live on therapy until they eventually die from something else.
The success of this strategy in a haematological cancer that has a relatively simple pathology gave a boost to the vision of turning other types of cancers from acute and potentially fatal diseases into manageable chronic conditions. But the strategy of ‘containment’ brought with it the burden of a lifetime on treatments that, while usually less toxic than traditional cancer therapies, still come with a variety of bothersome side effects. So once the initial euphoria over imatinib and successor TKIs died down, questions began to be asked about the possibilities of safely taking a short break from the treatment… or, where responses are really impressive, maybe even stopping altogether.
Those questions have been addressed in a series of clinical trials that are now opening up the prospect for many patients with CML to safely stop taking their drugs, subject to regular monitoring, with the option of restarting treatment with no added risk, where the blood markers indicate that is needed.
While not all patients who fit the criteria for stopping treatment actually choose to do so, many CML patients welcome the chance to live their lives ‘drug free’. The focus now is on deepening our understanding of why some patients who are eligible for stopping find that after a period they need to return to taking the TKIs, and on working out how to expand the proportion of patients who are able to stop for longer periods, and even indefinitely – with a possible role here for improving immune responses.
The success of this stopping strategy is being followed closely by clinicians and patient advocates in other disseminated cancer settings, including metastatic breast, prostate and lung – a topic that will be covered in a future article.
Stopping treatment
The idea that it might be possible for CML patients to stop taking the drug used to block the harmful kinase signalling emanating from their fused BCR-ABL genes first surfaced in 2007, a mere six years after imatinib was first approved. As the unprecedented impact of imatinib became clear – in terms of depth as well as duration of response – questions began to be asked about whether, in patients showing ‘deep molecular response’ (defined as a reduction of BCR-ABL protein in the blood to no more than 1/10,000th of the pre-treatment level), the disease might remain under control – in ‘treatment-free remission’ – without the need to continue drug treatment.
The stimulus for this question came from concerns among haematologists that, in addition to side effects disrupting patients’ day-to-day lives (fatigue, depression, disrupted sleep, and diarrhoea), they were beginning to see serious long-term consequences, including lasting damage to patients’ hearts, kidneys, lungs and liver. At the same time there were occasional case reports of women achieving prolonged remission after choosing to stop taking their TKIs to plan a pregnancy (TKIs are teratogenic).
“We were aware that the best way for our patients to achieve a normal life was to stop treatment”
The driving force behind the first TKI stopping trials in CML was François-Xavier Mahon, from the University of Bordeaux, who coordinated the first stopping trial. “We were aware that the best way for our patients to achieve a normal life was to stop treatment,” he says.
From the outset Mahon was confident that stopping imatinib would work in some CML patients. This conviction had been informed by his earlier experience leading a trial that explored the impact of stopping interferon alpha (the standard of care prior to imatinib) in CML patients experiencing severe side effects. The interferon alpha study, published in the JCO in 2002, showed that a small percentage of patients could achieve durable remissions after discontinuing the treatment.
Building the evidence
With TKIs, Mahon first undertook a pilot study stopping imatinib in 12 patients who had shown deep molecular response for more than two years. Published in Blood in 2007, the study showed that, at 18 months follow-up, half of them still had undetectable levels of BCR-ABL.
Next, Mahon persuaded colleagues in the French Group of CML (FI-LMC) to take part in the Stop Imatinib (STIM1) trial, which involved 100 patients from 19 participating institutions across France. To be eligible, CML patients needed to have been treated with imatinib and to be in sustained complete molecular remission (counterpart of a 4.5-log reduction of BCR-ABL) for more than two years. Mahon applied for a grant from the French Ministry of Health to fund the study, aware that convincing a pharmaceutical company to finance this project would be like asking turkeys to vote for Christmas.
Results, published in Lancet Oncology in 2010, showed that 12 months after stopping treatment, 41% of the 69 patients followed remained molecularly negative.
While the findings of that trial were very encouraging, more than half the participants had previously been treated with interferon alpha, which was no longer the standard of care. So Mahon launched a second trial, STIM2, which began enrolling patients in 2011, and involved 218 patients who had been treated with imatinib alone. The final results, published in 2019 in Clinical Cancer Research, showed that six months after stopping imatinib treatment, 52% of the patients showed no molecular signs of relapse, with that proportion falling only marginally by 24 months, to 50%.
“Most molecular recurrences occurred during the first six months of stopping, and after six months the probability was a rare event”
“Taking the two STIM studies together, we observed that most molecular recurrences occurred during the first six months of stopping, and after six months the probability was a rare event,” says Mahon. Crucially, he adds, patients who do experience molecular recurrence generally regain their previous deep molecular response after restarting TKI treatment. There have only rarely been cases of progression to advanced disease reported from any of the stopping trials.
This indicates that eligible patients face little risk in attempting to come off TKI treatment. It also opened up questions about whether patients who didn’t manage to achieve lasting treatment-free remission at first attempt might benefit from trying again.
This question was addressed in the French RE-STIM study, again coordinated by Mahon, which evaluated treatment-free remission in 70 patients having a second go at discontinuing TKI treatment after a first unsuccessful attempt. The results, which were published in Cancer in 2017, showed treatment-free remission rates of 48% at 12 months, 42% at 24 months, and 35% at 36 months.
While the success rate is encouraging, the reasons why second attempts can work after the first attempt has failed are not fully understood, says Susanne Saussele, from the University of Mannheim, Germany, who went on to work with Mahon as co-principal investigator of the EURO-SKI study. “It could be that the first treatment works like a vaccine, triggering the immune system when the patient is exposed for a second time to the TKI treatment. Or alternatively, the additional years of treatment may have finally killed off the CML stem cells,” she says.
“The reasons why second attempts can work after the first attempt has failed are not fully understood”
With the EURO-SKI (European Stop Kinase Inhibitors) study, Mahon and Saussele sought to widen patient enrolment beyond France, to the European stage. Their intention was to enrol a far larger population of patients to enable exploration of prognostic factors. Altogether 758 patients with CML were recruited from 61 European centres in 11 countries. To be eligible for enrolment they had to have taken imatinib or the second-generation TKI treatments (nilotinib or dasatinib) for at least three years and achieved a year of sustained deep molecular response. This time a small grant was provided by European LeukemiaNet for statistical analysis, with each of the individual investigators at the different country levels responsible for sourcing their own funding.
Results for the primary endpoint – molecular relapse-free survival – were published in Lancet Oncology in 2018, with Saussele as first author. They showed that 61% of patients were relapse-free at six months, dropping to 50% at 24 months. Outcomes were similar regardless of whether imatinib or second-generation TKIs were used.
Treatment-free remission in the EURO-SKI study
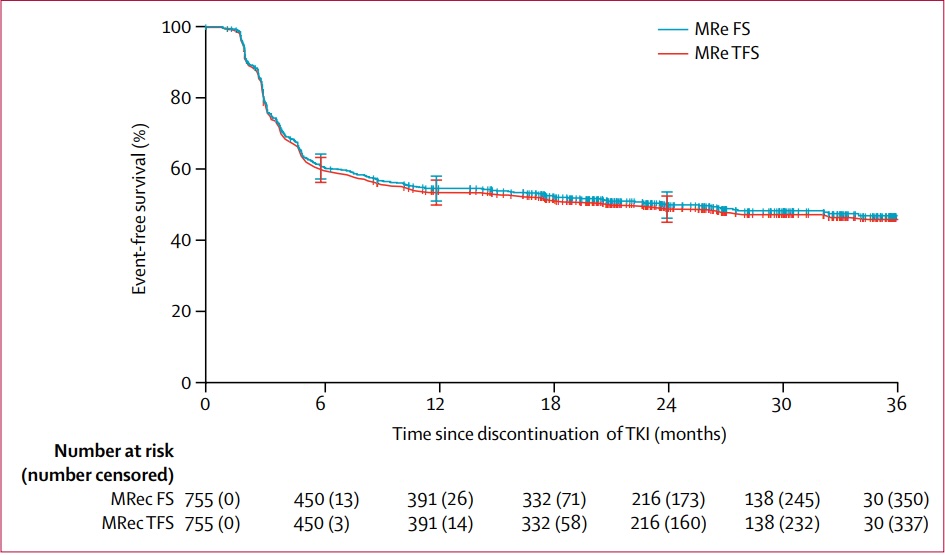
This study of more than 750 patients who came off treatment after taking a tyrosine kinase inhibitor (TKI) for at least three years and achieving deep molecular response showed 61% remained in treatment-free remission after 6 months, falling to 50% at 24 months. (13 of the patients opted to return to taking their TKI drugs despite remaining molecular relapse free. The red line includes this as ‘an event’.) Research efforts are now focused on understanding the factors that determine success in stopping treatment, and how to help patients succeed in stopping drug treatment if they fail at their initial attempt.Source: S Saussele et al (2018) Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncology 19:747–57 (figure 3). Republished with permission from Elsevier. © 2018 Elsevier Ltd
Results from the analysis of prognostic factors – a secondary endpoint – were presented in a second EURO-SKI paper, published in the JCO in March 2024. Duration of TKI treatment and duration of deep molecular response before stopping TKIs were identified as the main factors predicting major molecular response at six months. For major molecular response losses after six months, TKI treatment duration, percentage of blasts in the peripheral blood, and platelet count at diagnosis were significant predictive factors.
There was a statistically significant decrease in the burden of fatigue over time in patients in the 18–39 and 40–59 age groups
Other EURO-SKI analyses include a quality-of-life study, undertaken by Fabio Efficace, Head of the Health Outcomes Research Unit at GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto), Rome. The study, abstract S159, presented at the 2023 European Hematology Association Congress in Frankfurt, showed that patients aged between 18 and 39 years and 40 and 59 years typically reported greater benefits in quality of life across several functional and symptom outcomes than those in the 60–69 and 70+ age groups.
For example, there was a statistically significant decrease in the burden of fatigue over time in patients in the 18–39 and 40–59 age groups, but no statistically significant improvement in the two older age groups. “What this tells us is younger patients profit more from stopping treatment in terms of quality of life. The explanation is likely to be that older patients suffer from comorbidities, making beneficial effects of stopping TKIs less dramatic,” says Saussele.
Information on prognostic factors and quality of life can be invaluable in helping patients who have achieved the required molecular response make informed decisions about whether stopping treatment is right for them. “Ultimately whether patients who fulfil all the requirements stop is their individual decision. You need to discuss the side effects they’re experiencing on treatment, and factors like whether they want to travel and visit places where regular monitoring for BCR-ABL [required for discontinuation] may not be available,” says Saussele.
Potential downsides of going drug free
Coming off TKI treatment is a very welcome option for patients who fit the criteria. However, a significant proportion of the CML community currently has reservations about going down this road. A survey published in Leukemia & Lymphoma in 2018 reported that one in five CML patients who met the discontinuation criteria had no interest in stopping treatment.
In her clinical practice, Saussele has observed two distinct mind-sets towards stopping treatment. “There are patients diagnosed before the stopping trials, who have been conditioned to expect lifelong treatment and take some convincing. Then there are those diagnosed after the stopping trials, who from the outset have an ambition to stop,” she says.
Jan Geissler, a patient advocate who cofounded the CML Advocates Network in 2008, says that many patients like the reassurance of being able to take a daily tablet and forget about their CML. “They feel they’ve reached a safe haven, and don’t want to be exposed to new risks, like whether their disease would return and whether they’d be able to get back to the same level of response.”
Though the idea of being free of the tyranny of having to take a tablet every day is attractive, he adds, it comes with a new tyranny of needing regular blood tests to check the disease remains in deep molecular remission and they can safely stay off treatment.
These tests are done using polymerase chain reaction (PCR) to check BCR-ABL blood levels. Guidelines from the European Leukemia Network state that, immediately after stopping treatment, patients require a PCR test every four weeks, then after six months the intervals can increase to every six weeks, and at one year to every eight weeks. As Geissler, who stopped treatment in 2014, points out, this can be more than just a minor inconvenience.
Despite being treatment free for 10 years, Geissler does not think of himself as cured. “I am therapy free, but still have CML”
“Although it’s just a blood draw, the psychological burden of waiting for your results can’t be underestimated,” he says. “If you look at my PCR results, although close to the technical detection level of minimal residual disease, they’re continually up and down. You’re on a constant rollercoaster, where you’re anxious that your disease may have returned. Suddenly CML is at the back of your mind constantly, intruding into your day-to-day thoughts in a way it never did when I was on therapy.” Despite being treatment free for 10 years, Geissler does not think of himself as cured. “I am therapy free, but still have CML,” he says.
A survey undertaken by Geissler and colleagues from the CML Network, involving 1,016 CML patients across 68 countries, provides insights into the emotional strain that treatment-free remission can involve. The results, published in Leukemia in 2020, showed that at the time people are considering stopping, 22% said they would have liked to have received information on the psychological effects, and that during the first six months of stopping only 18% of respondents said they had discussed how to deal with psychological aspects with their doctor, 31% said they felt fear or anxiety before and/or after PCR monitoring tests, and 56% said they felt fear or anxiety at some point during the phase.
Such results point to the need for greater psychological support to be available to CML patients who come off TKI treatment. “Patients need to know they’re being taken care of and monitored closely, and that they are not part of an experiment with their lives,” says Geissler.
“Clinicians often talk about ‘relapse’, but this isn’t relapse, it’s a failure of stopping treatment”
Restarting treatment due to a molecular recurrence, the survey found, is a particularly challenging time, with 35% reporting feeling emotionally worse than before stopping. Patients need help to understand that molecular recurrence after a stopping TKIs is not a ‘failure of treatment’, says Geissler. “The vocabulary used is important. Clinicians often talk about ‘relapse’, but this isn’t relapse, it’s a failure of stopping treatment. Patients continue on the same pathway they were on before,” he says. It is a question of semantics, adds Geissler: a far less frightening – and more accurate – terminology would be to call it a ‘failure of being treatment free’.
Could this be the future?
One sure sign of levels of confidence in treatment-free remission strategies, at least in CML, is that some pharma companies serving this community are now funding their own stopping trials, including studies on dasatinib discontinuation (DADI, First-line DADI trial, D-STOP, and DASFREE) and on nilotinib discontinuation (STAT2, ENESTFreedom, ENESTSTop, and NILst). “With the concerns about the side effects of second generation TKIs, the dynamic has changed,” explains Mahon. “It’s become a marketing advantage for companies to be able to demonstrate that drugs don’t need to be taken for life.”
Within the academic community, research efforts are now focused on increasing the proportion of patients who are able to achieve treatment-free remission. “First, only about two thirds of patients taking TKIs can achieve molecular response levels considered the prerequisite to stop,” says Geissler, “Then, out of those who stop, around half will need to restart treatment. Taken together this means that only one third of the CML population will be able to stop successfully.”
What lies behind the divergent experiences of patients with similar levels of residual disease when stopping TKI treatment is now the ‘million-dollar question’ in CML. Why do some patients relapse, while others can remain treatment free for many years? Intriguingly, the answers seem to be pointing towards anti-tumour immune responses.
“We’re starting to understand that there are individual patient differences according to the number of quiescent stem cells resting in the bone marrow, and also how well the stem cells are controlled by their immune systems,” explains Saussele.
“Patients with CML needing to restart treatment had lower NK levels than those able to continue stopping”
Jani Huuhtanen, a postdoctoral researcher specialising in clinical haematology, who works in the lab of Satu Mustjoki at the University of Helsinki, has been investigating the immune responses of CML patients. In a study published in Leukemia, in January 2024, he undertook a retrospective analysis of samples taken from CML patients at diagnosis and then before and after TKI cessation, using single-cell RNA sequencing. He then compared the immune responses to those of healthy people and those with other cancers. Results showed that, compared to healthy people and those with other cancers, patients with CML had distinctive profiles of natural killer (NK) cells – a type of cytotoxic lymphocyte critical for immunity.
Furthermore, says Huuhtanen, “those patients with CML needing to restart treatment [after stopping] had lower NK levels than those able to continue stopping. The team also demonstrated that TIM3 (T-cell immunoglobulin and mucin domain 3), an immune checkpoint found on immune cells, was restraining NK cells. The findings raise the possibility that antibodies neutralising TIM3, like sabatolimab, might be given to patients failing treatment-free remission, to boost their levels of NK cells and improve stopping results.
Undoubtedly, there are other agents that could also be used to tip the immune balance in the patient’s favour. In an earlier study, published in the Journal of Clinical Investigation in 2022, Huuhtanen and colleagues showed that adding interferon alpha to the TKI dasatinib widened the number of immune cells expressed by patients with CML.
“Based on our studies, we believe the body’s own immune response to cancer cells must first be triggered to allow more patients to stop taking their medication,” says Huuhtanen.
The team at the University of Helsinki now plans to explore combining interferon alpha with different TKIs prior to stopping treatment, to identify optimal combinations. “We need to change the mindset from everyone being on therapy for ever to one where patients receive a fixed duration of therapy. If treatment discontinuation doesn’t work, we restart the drug and explore a more personalised approach, like changing the TKI or boosting their immune response,” says Huuhtanen. “If there’s a low probability of success, we need to introduce other strategies to be able to tip the balance in the patient’s favour.”
This is the first of two articles on building evidence around strategies for safely stopping treatment or taking treatment holidays for patients with well controlled disseminated disease. The second article will look at the efforts to build the evidence base in well controlled advanced breast, kidney and colorectal cancer.












