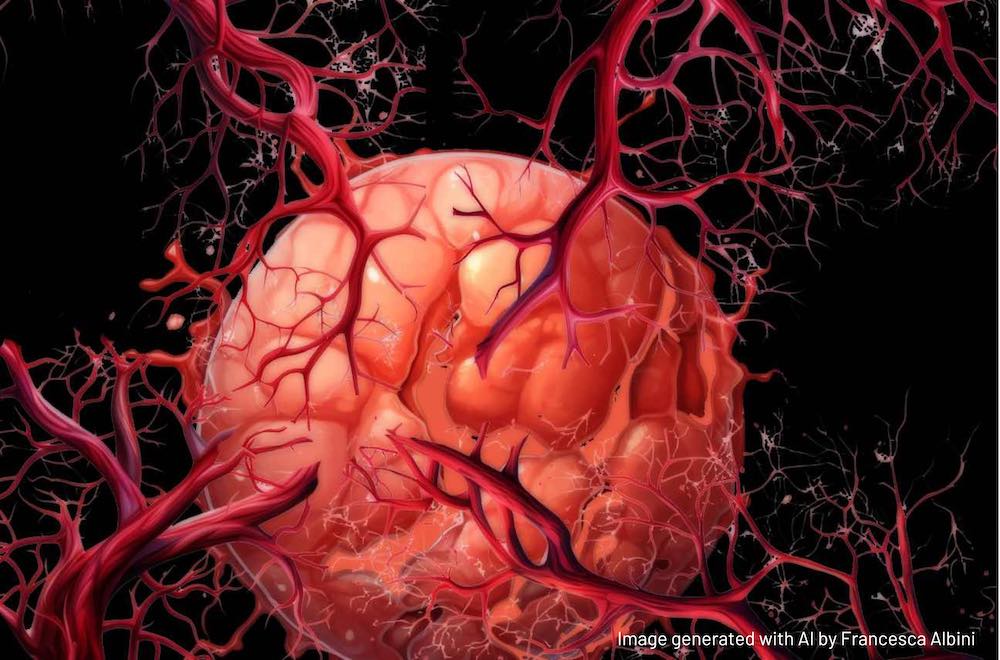
The human body is permeated by an extensive network of approximately sixty thousand miles of blood vessels – an intricately organised system designed for the precise and efficient distribution of oxygen and nutrients to all cells and organs, and for the removal of waste products, such as carbon dioxide. Nearly all metabolically active tissues are within a few hundred micrometres of tiny blood vessels called capillaries. On average, we have 19 billion of them. While a person’s vascular network is mostly formed by birth, it can change and adapt in response to physiological needs. The formation and growth of new blood vessels from pre-existing vascular structures is called angiogenesis. This fundamental biological process is active throughout our lives. It occurs during embryonic development, wound healing, menstrual cycle, exercise-induced muscle growth, organ maintenance, and adaptation to changing conditions.
When functioning properly, the physiological angiogenesis system controls the timing and location of blood vessel growth, to ensure that the body’s internal environment remains in a state of balance or homeostasis. But when there is an imbalance between pro-angiogenic factors, which promote blood vessel growth, and anti-angiogenic factors, which inhibit it, pathological angiogenesis occurs. This imbalance is a common feature in numerous health conditions including cancer, as well as age-related macular degeneration, diabetic retinopathy, rheumatoid arthritis, psoriasis, atherosclerosis, endometriosis, stroke, chronic wounds and liver cirrhosis.
In their seminal publication on the Hallmarks of Cancer, published in 2000, ‘sustained angiogenesis’ was identified by Douglas Hanahan and Robert Weinberg as one of the six (since expanded to eight) core ‘acquired capabilities’ shared in common by most, if not all, cancers. This was almost 75 years after a potential connection between cancer and changes in the vascular system had first been flagged up, in 1927 by an anatomist at Johns Hopkins University, and around 30 years after a biologist and paediatric surgeon at Boston’s Children’s Hospital, Judah Folkman, first published the hypothesis that cancer cells are dependent on being able to generate an adequate blood supply, and that finding ways to deprive them of that ability could be a fruitful therapeutic strategy.
It took until 2004 for that strategy to be developed and reach the clinic, with the approval of the first angiogenesis inhibitor, bevacizumab, in 2004 – initially for use in colorectal cancer. Today oncologists have several anti-angiogenesis agents to choose from, including monoclonal antibodies, small molecules, and immunomodulatory drugs, each with distinct mechanisms of action, approved for use in more than 25 types of cancer.
Angiogenesis and cancer: how the story unfolded
Suggestions of a potential connection between cancer and changes in the vascular system first emerged in the early 20th century. In 1927, an article by the anatomist Warren Lewis, on ‘The vascular patterns of tumors’, which was published in the Bulletin of the Johns Hopkins Hospital, provided evidence that different tumour types in rats had distinct vascular structures, indicating that the tumour environment influences blood vessel growth. In 1928, J.C. Sandison, an anatomist at the University of Pennsylvania Medical School, introduced a transparent chamber for observing living tissues, enabling progress in understanding how tumours affect blood vessels. Ten years later, Gordon Ide and colleagues used Sandison’s technique to study the relationship between transplanted rabbit carcinomas and their blood supply. Their findings, that tumour growth was associated with rapid blood vessel formation, were published in 1939 in the American Journal of Roentgenology.
This line of research was further pursued by Glen Algire, at the US National Cancer Institute, who conducted experiments that suggested that blood vessels are drawn towards tumours. His research team implanted transparent plastic chambers in mice and placed tumour samples in them. In a paper published in 1945, in the Journal of the National Cancer Institute, Algire observed that blood vessels grew directly towards the tumours, at an even faster rate than was seen in normal wounds. The findings led him to speculate that tumour cells release a substance that stimulates the rapid growth of new blood vessels – the first time such a mechanism had been proposed within the specific context of cancer. However, little follow-up or attention was given to Algire’s findings, and he himself does not seem to have pursued further research in this area.
The findings led him to speculate that tumour cells release a substance that stimulates the rapid growth of new blood vessels
The idea that chemical mediators may play a vital role in biology had been around for some 20 years by the time Algire speculated on what lay behind the rapid growth of new blood vessels around tumours. One of the key milestones in the development of this concept was the discovery made by the English pharmacologist Sir Henry Dale and Scottish virologist Patrick Laidlaw in the 1920s of histamine as a chemical mediator in allergic reactions. In 1948 – three years after Algire published his theories over the cause of vascularisation surrounding tumours – ophthalmologist Isaac Michaelson proposed something similar in relation to proliferative diabetic retinopathy, an eye condition characterised by abnormal new blood vessels that grow on the surface of the retina. In an article published in the Transactions of the Ophthalmological Society UK, he suggested that the neovascularisation may be caused by a diffusible ‘Factor X’ produced by the retina.
Researchers began to hypothesise that tumours might produce similar factors to stimulate blood vessel growth. But it wasn’t until 1968 that the first direct experiments demonstrating the release of angiogenic factors by tumour cells were conducted. Melvin Greenblatt and Philippe Shubi, and Robert Ehrmann and Mogens Knoth and others, used a transparent chamber to show that tumour cell transplantation promoted blood vessel proliferation, even when a filter separated the tumour and the host.
From speculation to therapeutic strategy
So far, much of this work had been driven by a scientific interest in learning about the mechanisms involved in angiogenesis. This all changed when Judah Folkman, a young surgeon from the Harvard affiliated Boston Children’s Hospital, entered the scene. Folkman had been drafted to a two-year stint at the National Naval Medical Center in Bethesda. Along with his colleague Fred Becker, he had been tasked with investigating potential blood substitutes suitable for emergency use on naval ships.
Their approach was to perfuse isolated organs with their candidate blood substitutes – which consisted primarily of haemoglobin solutions – to see how long they could remain viable. As a further test of the properties of these perfused organs, they implanted small explants of murine melanomas into the perfused thyroids.
Folkman observed that, when tumours formed in vitro within the gland, their growth was restricted. However, when the same cancer cells were implanted in a living mouse, the tumours grew vigorously. It was this finding that first led Folkman to wonder whether cancer growth could be halted by interrupting its blood supply. He believed there must be a pro-angiogenic factor that could be identified, and an opposite anti-angiogenic factor which would stop or reverse the process. The prevailing cancer treatments at the time involved chemotherapy and radiation, which indiscriminately killed both cancerous and non-cancerous cells. The possibility of finding a new way to fight cancer was therefore very appealing.
He believed there must be a pro-angiogenic factor that could be identified, and an opposite anti-angiogenic factor which would stop or reverse the process
During the following decade, Folkman kept on thinking about those experiments and what they could mean for the treatment of cancer. In 1971 he published an article in the New England Journal of Medicine, in which he proposed that tumour cells and vascular endothelial cells within a neoplasm may constitute a highly integrated ecosystem. It was the first time the concept of the tumour microenvironment had been proposed in such an explicit way. Folkman suggested that endothelial cells may be switched from a resting state to a rapid growth phase by a chemical signal from tumour cells. He called it ‘tumour angiogenesis factor’ (TAF). He also coined the term ‘anti-angiogenesis’, to mean the prevention of new vessel sprouts from penetrating into an early tumour implant.
The ‘angiogenic switch’ – a significant milestone in tumour progression
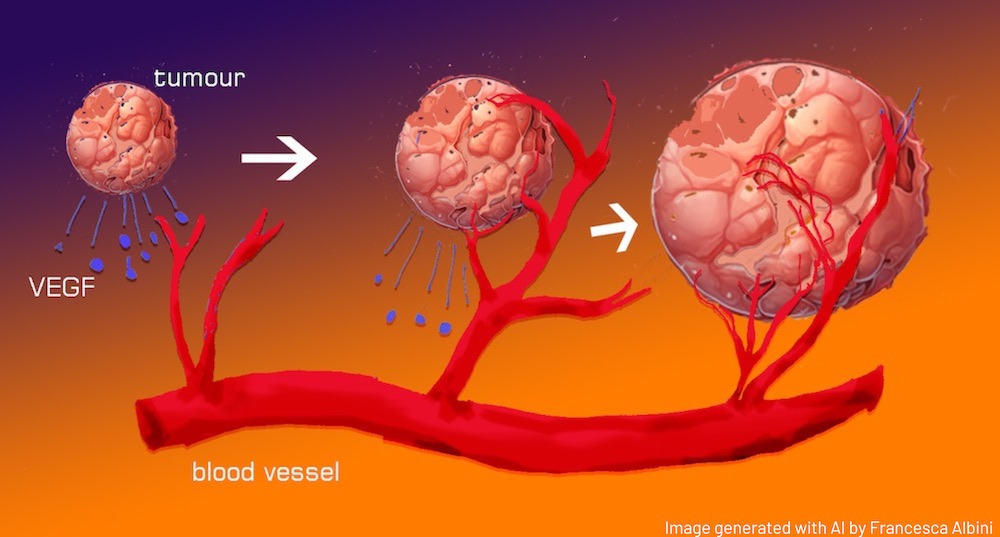
In the late 1980s, the term ‘angiogenic switch’ was coined to describe a point in tumour growth where the balance of factors influencing blood vessel formation tips in favour of the development of new blood vessels. This shift is pivotal because it transforms a relatively stagnant, avascularised hyperplasia (a dense, but blood vessel-lacking cell overgrowth) into a rapidly expanding tumour that is supported by a network of blood vessels, which provides it with nutrients and facilitates its growth. This transition typically happens relatively early in the development of a cancerous growth and is a significant milestone in tumour progression.
The importance of this switch should be understood in the context of evidence showing that the existence of tiny tumours that never progress during a person’s lifetime is quite commonplace. Autopsy studies conducted on bodies of individuals who never received a cancer diagnosis during their lifetimes reveal that approximately 40% of women aged between forty and fifty harboured microscopic tumours in their breast tissue; nearly 50% of men aged fifty to sixty had minute cancerous growths in their prostate glands; and nearly 100% of individuals over the age of seventy exhibited microscopic cancers in their thyroid glands.
These tumours arise when normally healthy cells undergo genetic mutations during cell division or due to environmental factors. On a daily basis, our cells may experience up to ten thousand DNA errors, making the development of cancer a common and almost unavoidable occurrence. While these errors may result in microscopic cancers that pose no immediate threat, our body has a remarkable defence mechanism aimed at restricting their growth by depriving them of essential blood supply and nutrients. However, if these tiny cancers gain the capacity to elude the body’s natural defences and trigger the formation of new blood vessels, they can evolve into more aggressive and potentially life-threatening malignancies. Blood vessels also constitute the major route of tumour cell dissemination, leading to the formation of metastases.
The 1970’s and 80’s saw a series of new discoveries that progressed this area of research. In 1973–74, Armelin and Gospodarowicz independently detected a factor within the bovine pituitary gland that exhibited a potent mitogenic effect on fibroblasts, endothelial cells, and chondrocytes. It took the name of fibroblast growth factor (FGF). In 1983, Ann Dvorak and Donald Senger discovered and isolated a protein involved in angiogenesis, which they named ‘vascular permeability factor’ (VPF). In 1984, Shing, Folkman, and colleagues, discovered a factor in tumours that stimulated the growth of capillary endothelial cells in vitro and induced the development of new blood vessels in live chicks. Purifying this substance was made easier by its strong affinity to heparin. In 1986, Presta, Moscatelli, and colleagues, isolated an angiogenic factor from human placenta and human hepatocellular carcinoma cells, capable of triggering DNA synthesis, cell motility, and proteases production in capillary endothelial cells. It also induced angiogenesis in vivo. By studying its amino acid sequence, they found that this substance was human basic fibroblast growth factor (bFGF).
Then, in 1989, Napoleone Ferrara and his team cloned, sequenced, and characterised the ‘vascular endothelial growth factor’ (VEGF), which was found to be the same as the ‘vascular permeability factor’ identified six years previously. A pivotal point in the history of VEGF came in the 1990s, when it became identified with the mysterious ‘Factor X’ that Isaac Michaelson proposed in 1948 as playing a role in proliferative diabetic retinopathy. In 1993, a significant discovery demonstrated that a monoclonal antibody targeting VEGF had a remarkable effect in suppressing tumour growth in living organisms. This breakthrough paved the way for the development of bevacizumab, a humanised version of this anti-VEGF antibody. Bevacizumab received FDA approval in 2004 for use as a first-line therapy in metastatic colorectal cancer.
VEGF – what it is, what it does
Vascular endothelial growth factor is a family of growth factors that bind to receptors on the surface of endothelial cells, which line the inside of blood vessels. There are several VEGF isoforms, of which VEGF-A is the most well-known and important within this context.
VEGF exerts its effects by binding to VEGF receptors (VEGFRs) on the surface of endothelial cells. There are several receptors and co-receptors (such as neuropilins), with the tyrosine kinases VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1) being the most well-studied. VEGFR-2 is particularly important for mediating the angiogenic effects of VEGF.
When VEGF binds to its receptors on endothelial cells, it triggers a series of intracellular signalling events that promote endothelial cell proliferation, migration, and survival. This activation includes changes in gene expression and cellular behaviour. As the endothelial cells become activated, some differentiate into ‘tip cells’. These are located at the leading edge of the growing blood vessel and play a crucial role in guiding the formation of new vessels. They extend long, finger-like projections called filopodia, which probe the surrounding tissue for guidance cues and create a path for the new vessel. The filopodia sense gradients of VEGF and other factors to navigate towards the source of the pro-angiogenic signals.
Behind the tip cells, endothelial cells differentiate into ‘stalk cells’, which proliferate and form the core of the new blood vessel. They provide structural support and help elongate the vessel. The stalk cells eventually organise into a tube-like structure, and a lumen forms within the vessel, which will carry blood once the vessel is fully matured. As tip cells continue to extend and branch, they create a network of interconnected vessels through a process called anastomosis.
The newly formed blood vessels mature and stabilise. Pericytes and smooth muscle cells are recruited to the vessel walls to provide structural support and regulate blood flow. In physiological angiogenesis, once the tissue’s oxygen and nutrient needs are met, production of angiogenic factors is downregulated and the newly formed blood vessels become quiescent or regress.
A new pillar of anti-cancer therapy
The following 20 years have seen multiple anti-angiogenesis therapies approved for treating more than 25 different types of cancer – not just monoclonal antibodies, but also small molecules, and immunomodulatory drugs, with distinct mechanisms of action. Most are directed to intercept the signalling between angiogenic factors and their receptors or mediators.
Monoclonal antibodies, for instance, were designed to specifically recognise and attach to VEGF, preventing it from activating the VEGF receptor and effectively blocking the formation of new blood vessels. Small molecules were designed for binding to VEGF or its receptors, as well as to other cell surface receptors and proteins involved in the signalling pathways responsible for blood vessel growth. Upon binding, these molecules impede the kinase activity of VEGFR-2, a receptor tyrosine kinase. Normally, this kinase activity adds phosphate groups to specific tyrosine amino acids, including those within VEGFR-2 itself and downstream signalling proteins. By inhibiting VEGFR-2 from carrying out this function, these small molecules interfere with the signalling pathways that regulate blood vessel growth, ultimately leading to a reduction or inhibition of angiogenesis.
Immunomodulatory drugs have dual roles. These drugs can hinder the development of new blood vessels while simultaneously enhancing the immune response against cancer cells.
Angiogenesis inhibitors have shown positive results in cancer therapy, especially when used in conjunction with other drugs. Colorectal cancer, the indication for which the first such inhibitor was approved in combination with chemotherapy, remains its most successful anti-cancer application. However, it is in the ophthalmology setting that targeting VEGF has been a winning therapy, preventing and curing macular degeneration.
Anti-angiogenic treatments also come with potentially serious side-effects. Treatment with VEGF-targeting angiogenesis inhibitors may result in bleeding, arterial clots, high blood pressure, delayed wound healing, posterior leukoencephalopathy syndrome, gastrointestinal perforation, and fistulas. These treatments are also associated with fatigue, diarrhoea, disruptions in thyroid function, changes in the skin of hands and feet, and alterations in hair texture or growth. One drawback of the strategy is that, to be effective in impeding expansion of tumours, a prolonged inhibition of vascular recruitment is required, defined as ‘angiostasis’ by Hanahan and Folkman. As extended treatment regimens are necessary, it is important to find and characterise angiostatic compounds that exhibit minimal to no toxicity.
At a more general level, the angiogenesis era has expanded scientific horizons, shifting the focus from solely targeting cancer cells to addressing the broader context of the tumour microenvironment. In this regard, the advent of immunotherapy, particularly through the use of checkpoint inhibitors, has presented distinct advantages in comparison to anti-angiogenesis therapies. These advantages include a broader applicability to different cancer types, the potential for long-term responses and immune memory, adaptability to genetic mutations, fewer severe side effects, and the ability to be combined with other treatments. However, new clinical trials are underway that highlight the potential of a dual strategy that simultaneously addresses tumour blood vessels and the immune system, offering a promising approach to cancer treatment. This integrated approach has demonstrated substantial benefits in preclinical models, showing an association with enhanced T cell infiltration into the tumour microenvironment, and fostering a more robust and targeted immune response against cancer.
With the collaboration of Francesca Albini
The cardiovascular system – what we’ve learned since our ‘cave man’ days
The history of our understanding of the cardiovascular system is long and fascinating. As we gather from cave paintings, the Palaeolithic bowmen already knew that they should aim at the heart of the prey for an efficient kill. Read more…