It is often said in medicine that the only true constant in facing life-threatening diseases is change. Nowhere is this more evident than in oncology, where advances in chemotherapy, targeted therapies, immunotherapies, and other novel agents continue to spark hope. Yet people still ask: Why isn’t there a universal cure for cancer? Perhaps the question itself is misguided. We don’t seek a single treatment for every type of infection, after all. Cancer, similarly, is not one disease but a constellation of disorders with different genetic and biological drivers.
Colorectal cancer (CRC) illustrates this complexity. Decades ago, metastatic CRC was nearly untreatable. Then, chemotherapy with 5-fluorouracil (5-FU) and platinum-based drugs extended survival and cured some early-stage cases. Targeted therapies offered additional gains, but most advanced diseases remained beyond cure. Dogma held that metastatic CRC is incurable. Yet as scientific knowledge evolves, so do our certainties.
The last two decades saw immunotherapy generating extraordinary optimism in some cancers, where complete responses or long-term remissions emerged. In CRC, that success hinges on how tumors repair DNA damage. Deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H) leads to enough mutations to draw the immune system’s attention. However, nearly 80% of CRCs are microsatellite-stable (MSS) and lack these mutation profiles, rendering them invisible to standard immunotherapies. The challenge, then, is clear: how can we bring immunotherapy benefits to this majority?
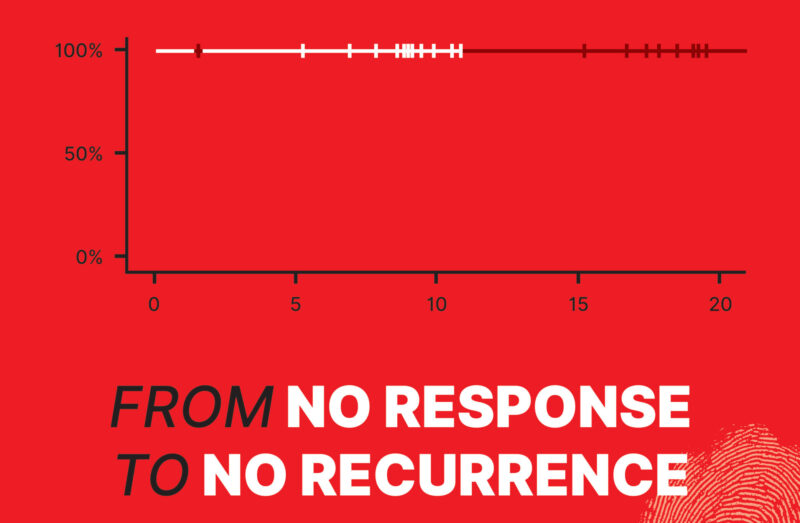
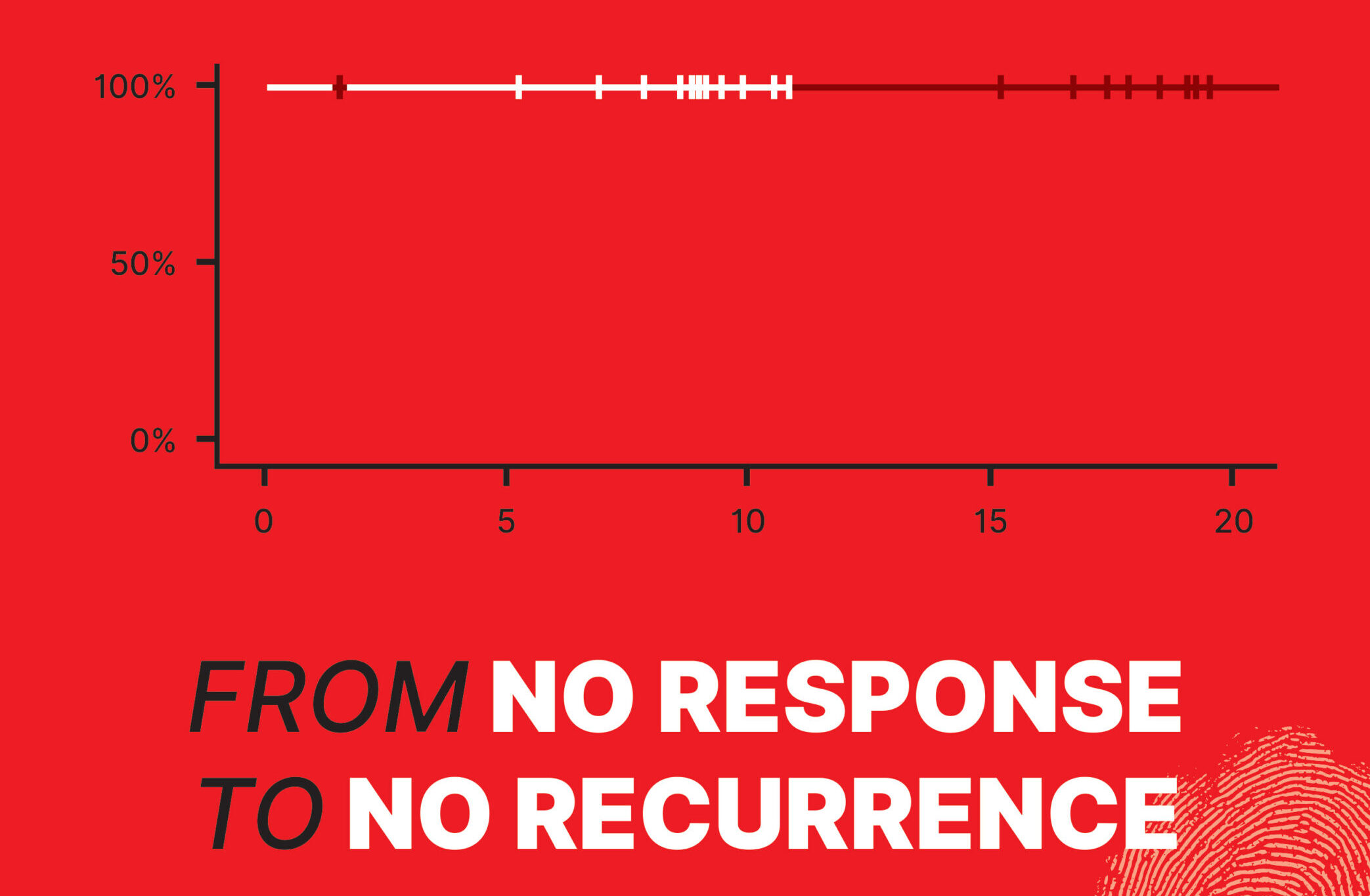
In this shifting landscape, botensilimab—a next-generation, Fc-enhanced CTLA-4 inhibitor developed by Agenus Inc.—has fueled cautious optimism. Paired with balstilimab, a PD-1 inhibitor, it forms a dual approach designed to penetrate the traditionally unresponsive MSS population. In two independent neoadjuvant trials (UNICORN and NEST) involving more than 80 patients, botensilimab plus balstilimab produced responses once considered unattainable in MSS colon cancer. UNICORN, a Phase 2 study in Italy with 56 patients, reported a 93% pathological complete response (pCR) rate in the dMMR/MSI-H subset—and, more surprisingly, a 29% pCR and 36% pathological major response rate in MSS tumors. Only one surgery was delayed due to side effects. Meanwhile, data from other U.S. studies indicated zero recurrences and 100% ctDNA negativity at 18.2 months (NEST1 trial) and 8.98 months (NEST2 trial), hinting that a substantial fraction of MSS patients might achieve deep remission before ever entering the operating room. This promising data inspired the cover image of this issue—a flat curve from NEST1 and NEST2 trials with a 100% recurrence-free status at the current follow-up.
Encouraging data extends beyond the neoadjuvant setting. A randomized Phase 2 trial (NCT05608044) with 234 patients who had refractory MSS metastatic CRC compared botensilimab plus balstilimab to regorafenib or trifluridine/tipiracil. The new combination achieved a 19% overall response rate (ORR) and a 55% disease control rate (DCR), while today’s standard of care arm showed 0% ORR. Around 70% of these responses were ongoing at the time of analysis. Although 89% of participants reported treatment-related side effects and 12% discontinued, such figures can be acceptable in a population with few remaining options. Even a 10–20% chance of meaningful tumor reduction can be life-changing.
Preliminary findings also suggest that combining botensilimab/balstilimab with standard chemotherapy (FOLFOX) and targeted therapies like bevacizumab may yield response rates as high as 71% in MSS disease. If confirmed, this synergy could expand patient eligibility and improve outcomes without incurring untenable toxicity. While “paradigm shift” is often overused, the possibility of immunotherapy success in MSS CRC could earn that label. A once-impervious subtype is now within reach, challenging the long-held belief that MSS disease is untouchable by immunotherapies.
Such breakthroughs do not come without risks. Immune-mediated toxicities can affect various organs, and not every patient responds. Yet even incremental advances matter. Because “progress” can never be purely numerical in oncology. A few extra months—or years—can mean a father witnessing his daughter’s graduation or a mother holding her first grandchild—profound reminders that “statistics on a chart” translate into something more valuable – human experiences. In the neoadjuvant setting, a substantial response might also spare patients from disfiguring or life-altering surgeries, underscoring that quality of life is at stake as much as survival. Historically, medicine has repeatedly revised its certainties: advanced breast cancer was once universally fatal, then new therapies extended remission for many; MSI-H CRC seemed off-limits to immunotherapy until dramatic remissions surfaced. Today, MSS CRC stands poised on the cusp of a similar shift: from “no response” to potentially “no recurrence.”
The next steps—regulatory approvals and Phase 3 trials—are critical. Can botensilimab and balstilimab help patients at every stage, from first-line metastatic to second-line salvage? Will neoadjuvant use boost cure rates further? Who benefits most? The answers could reshape the care landscape for thousands of patients, paving the way for organ-preserving strategies and better long-term outcomes. While no one expects instant transformation, still, every piece of evidence that brings MSS CRC toward improved outcomes signals that dogmas can indeed be dismantled. Progress often begins when we dare to question what we think we know.
Although the data are encouraging, botensilimab remains investigational, and further studies are needed to confirm its efficacy while carefully balancing its benefits against potential toxicities. Yet its overarching principle—that even deeply ingrained assumptions can be overturned—is already making waves. Data from UNICORN and NEST, plus the randomized Phase 2 trials in advanced disease, hint at a viable path for MSS CRC patients once deemed out of reach. In oncology, major leaps often start as bold questions that eventually topple conventional wisdom. Should botensilimab deliver on this early promise, it will join that remarkable lineage. For now, it stands as a powerful reminder that medicine’s “facts” can change the moment new evidence emerges. “Incurable” can become “durable remission,” and, for some, “no response” may yet turn into “no recurrence.”
Disclaimer
Botensilimab and balstilimab remain under investigation and are not yet FDA-approved. The findings described here are based on interim data. Patients should consult with qualified healthcare providers about current treatment options. This overview is for informational purposes and should not replace professional medical advice.