A CAR T-cell therapy first-in-human study demonstrated early signs of efficacy for patients with a range of solid tumours, including testicular and ovarian cancers. The study, presented at the American Academy of Cancer Research (AACR) 2022 Annual Meeting (8-13 April 2022), held in New Orleans, combined two innovative approaches, autologous CAR T-cell therapy and a CAR T-cell amplifying RNA vaccine to improve persistence and functionality of cells.
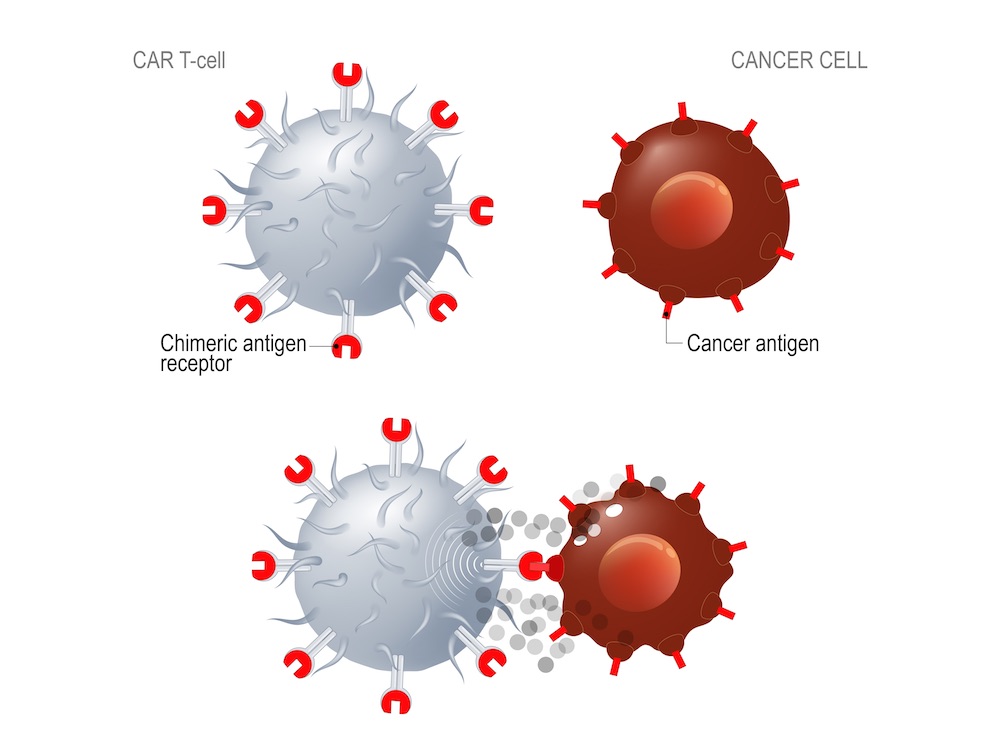
The process of chimeric antigen receptor T-cell (CAR T-cell) therapy involves harvesting immune cells from patients and genetically engineering them to express synthetic receptors that recognise proteins specific to cancer. Modified cells are manufactured in large quantities before being infused back into the patient. While CAR T-cell therapy has revolutionised treatment of haematologic malignancies, application in solid tumours has proved more challenging. “One of the main limitations is that most of the proteins present on solid tumours that could be used as targets are also found at low levels on normal cells, making it difficult to specifically direct the CAR-T cells against tumour cells and spare the healthy ones,” explained study presenter John Haanen, a medical oncologist from the Netherlands Cancer Institute, Amsterdam.
The new therapy, BNT211, is composed of two separate drug products. First, a CAR T-cell therapy targets the antigen Claudin-6 (CLDN6), a tight junction molecule known to be upregulated in at least 20 types of human cancer, but silenced in healthy tissue. Second, the CAR T-cell Amplifying RNA Vaccine (CARVac) is administered to promote CAR T-cell activation in vivo.
The Phase I/II open-label trial was undertaken in16 patients with advanced CLDN6-positive tumours that did not respond to, or relapsed after, prior therapy (testicular cancer n=8, ovarian cancer n=4, endometrial cancer n=1, fallopian tube cancer n=1, sarcoma n=1, and gastric cancer n=1). The study took place in two parts, with separate BNT211 dose escalations for monotherapy (part 1), then its combination with CARVac, administered every two to three weeks for up to 100 days after the CAR T –cell infusion, followed by maintenance doses every six weeks (part 2).
Results showed that, 10 to 17 days after infusion, all 16 subjects demonstrated ‘robust ‘ engraftment with modified CAR T-cells.
At the first efficacy assessment, six weeks post infusion, six out of 14 evaluable patients (43%) showed a partial response, five patients had stable disease with shrinkage of target lesions, one patient showed no change from baseline and two patients showed disease progression. The overall response at six weeks was 67% for testicular cancer (four of six patients) and 50% for ovarian cancer (two of four patients). Four of the responders received CAR T-cell therapy on its own, while two also received the mRNA vaccine.
At 12 weeks, four of the six patients who had achieved response showed a “deepening and durable response”, and at 18 weeks one patient demonstrated a complete response. All four patients with testicular cancer treated with the higher dose level had disease control and three of these patients showed objective responses.
Antitumour activity tended to be higher at the higher CAR T-cell dose, and when combined with the vaccine, with four of the five patients in the CARVac combination group showing a partial response. However, in some patients there was evidence of the treatment effect wearing off said Haanen.
Approximately 40% of patients developed cytokine release syndrome (CRS) ‒ a known complication of CAR T-cell therapy, which resolved after treatment with the anti-inflammatory drug toclizumab. Patients who received the vaccine had manageable flu-like symptoms, and some patients exhibited asymptomatic elevated lipase levels ‒ a possible sign of pancreatic toxicity.
“The infusion of CLDN6 CAR T, alone or in combination with CARVac is safe and holds promise for patients with CLDN6-positive cancers. CLDN6 was never targeted before with cellular therapy, but in our study this approach is already showing efficacy that may be better than data from other CAR T trials in solid tumours,” said Haanen. “While the data are very early, it is remarkable that all patients with testicular cancer showed clinical benefits at dose level 2, and the responses we have observed can be deep, including one ongoing complete remission.”
The discussant, Vincent Lam, from Johns Hopkins University in Baltimore, said, “These updated results are an important and exciting advance in a rapidly growing field. For the first time, CLDN6 is validated as a novel CAR T target with promising clinical activity in testicular and ovarian cancer.”
He added, however, that CAR T-cell therapy did not yet appear to show consistent enhancement. “Remaining questions include: what is the optimal dose, timing and duration of CARVac and the optimal setting, and whether the effect could be even more apparent in a lower–antigen-density tumour.” The lipase elevation, he speculated, might represent an on target-tumour effect on the pancreas, which might suggest a possible role in pancreatic cancer.
BioNTech, the company developing the regimen, said that additional data from the ongoing trial will be available later in the year.