Among patients with cancer cachexia and elevated levels of the cytokine GDF-15, inhibiting GDF-15 with ponsegromab resulted in increased weight gain. The study, published in the New England Journal of Medicine, 14 September, showed that in comparison to placebo, patients taking ponsegromab had reduced cachexia symptoms and improvements in appetite, overall physical activity, and skeletal muscle mass.
“Collectively, these results point to the potential for ponsegromab as a novel targeted therapy to address the unmet medical needs of patients with cancer and cachexia,” John Groarke, the corresponding author on the paper from the Pfizer Internal Medicine Research Unit in Cambridge, Massachusetts, tells Cancerworld.
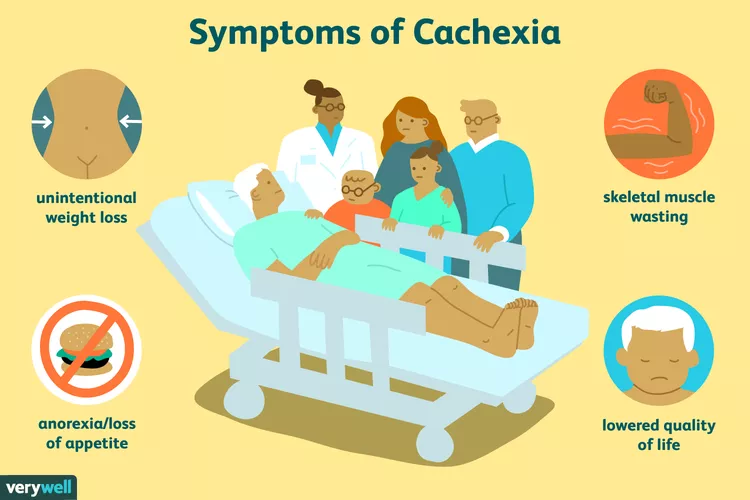
Cachexia (wasting syndrome) is common among patients with advanced cancer and can lead to weight loss, muscle wasting, reduced quality of life, functional impairment, and reduced survival. With no approved medications for the treatment of cancer cachexia in the US or Europe, treatment options have been limited. One potential target is GDF-15 (cytokine growth differentiation factor 15), a member of the TGFβ superfamily, which is a global regulator of the body’s response to stress. Elevated GDF-15 concentrations have been associated with weight loss and poor outcomes in patients with certain types of cancer. In mouse tumour models, inhibition of GDF-15 has been shown to reverse weight loss and improve survival.
Ponsegromab is a potent, highly selective, humanised monoclonal antibody that binds to circulating GDF-15, thereby inhibiting interaction with the GDF-15 receptor. In a small open-label, Phase 1b study, involving 10 patients with cancer cachexia and elevated circulating GDF-15 levels, published in Clinical Cancer Research in February 2024, use of ponsegromab was associated with improved weight, appetite, and physical activity along with suppressed levels of serum GDF-15.
For the current Phase 2 randomised, double-blind trial, between February and December 2023, 187 patients with cancer cachexia and elevated serum GDF-15 levels (≥1500pg/mL) were randomised 1:1:1:1 to receive three injections of ponsegromab at a dose of 100mg, 200mg, or 400mg, or to receive placebo, administered subcutaneously every four weeks. Of the patients (median age 67 years, 63% men), 40% had non-small-cell lung cancer, 32% pancreatic cancer, and 29% colorectal cancer. To be eligible, patients were required to have an involuntary weight loss of >5% within the previous six months or of >2% if their BMI was <20kg/m2 (as defined in the International Consensus Definition of Cachexia). Additional inclusion criteria were: a serum GDF-15 level of at least 1500pg/mL; an Eastern Cooperative Oncology Group performance-status score of 3 or less (on a scale of 0–5, with higher numbers reflecting greater disability); and a life expectancy of at least four months.
Results at 12 weeks show the increase in weight from baseline was 1.22kg in the 100-mg group (95% credible interval 0.37–2.25); 1.92kg in the 200mg group (95% credible interval 0.92–2.97); and 2.81kg in the 400mg group (95% credible interval 1.55–4.08) – all P<0.05 in comparison with placebo. Ponsegromab, the authors comment, was even associated with weight gain in patients with the most severe weight loss.
Among all the patients in the study, 39% in the 200mg ponsegromab group, 28% in the 400mg group, 26% in the 100mg group and 21% in the placebo group reported no appetite loss at baseline.
Patients in the 100-mg and 400-mg ponsegromab groups showed improvements from baseline as compared with the placebo group at 12 weeks regarding the Functional Assessment of Anorexia Cachexia Treatment (FAACT)-Anorexia Cachexia Subscale and the FACCT 5-Item Anorexia Symptom Scale. No material differences in the score on either scale were observed in the 200-mg ponsegromab group relative to the placebo group.
Patients in the 400-mg ponsegromab group showed increased overall activity at 12 weeks compared with the placebo group, with a difference of 72 minutes per day (95% credible interval 37–107) with respect to non-sedentary physical activity.
Adverse events of any cause were reported in 70% of patients in the ponsegromab groups versus 80% in the placebo group. Serious adverse events from any cause occurred in 22–40% of patients in the ponsegromab groups and in 24% of those in the placebo group.
“Here we report improvements across weight and body composition, quality of life, and physical function driven by a single pharmacologic intervention directed against GDF-15,” write the authors.
The results, they add, provide the first conclusive demonstration that GDF-15 represents a common driver of cachexia across different malignant solid tumours, thereby establishing GDF-15 as a therapeutic target. “Furthermore, the ponsegromab-mediated increase in nonsedentary physical activity may represent clinically meaningful functional improvement by enabling patients to complete important daily activities, such as showering, dressing, and light household activities,” they write. The placebo-like safety profile, add the authors, may differentiate ponsegromab from other agents used in cancer cachexia.
Additional studies, write the authors, are needed to determine the appropriate timing for ponsegromab initiation along the cancer cachexia continuum.
Based on these results, Pfizer is discussing late-stage development plans with regulators with the goal of starting registration-enabling studies in 2025.
Elevated circulating GDF-15 levels have been reported in other diseases including heart failure, chronic kidney disease, and chronic obstructive pulmonary disease. “Our findings of definitive disease modification associated with GDF-15 inhibition highlights the broad therapeutic potential for this mechanism of action, with possible implications for diseases beyond cancer cachexia,” write the authors. Ponsegromab is currently being investigated in a Phase 2 study in patients with heart failure and elevated serum GDF-15 concentrations.
Opening illustration from Verywell, illustration by JR Bee. Source: Verywell Health