The advent of chimeric antigen receptor T cell (CAR T cell) therapy generated great excitement in the field of onco-haematology. Clinical trials have shown remarkable results in patients with relapsed/refractory B cell malignancies, and two CAR T cell products have been approved in the US and in Europe. Efforts are now underway to extend this approach to other haematological malignancies and even to solid tumours.
Yet the clinical trials that have shown the huge potential of CAR T cells, at the same time revealed their unique toxicities. Cytokine release syndrome (CRS) and immune effector cell associated neurologic syndrome (ICANS) are two key toxicities associated with CAR T cell therapy, though other adverse events also occur and need to be taken into consideration in clinical practice.
The morbidity associated with the side effects is not irrelevant. Though generally reversible, on rare occasions it has led to death. Oncologists need to know how to diagnose and manage toxicities related to CAR T cell therapy.
According to published studies, after CD19-targeted CAR T cell therapy, CRS was experienced in different grades by 37%–93% of patients with lymphoma and 77%–93% of patients with leukaemia. Rates of any grade ICANS were, respectively, 27%–67% and 40%–62%. In the early clinical trials, approximately half of the patients needed intensive care management.
The task of developing optimal strategies for managing these toxicities has been hindered by the considerable variation in the way they have been assessed and graded across clinical trials and across institutions. In an attempt to address this problem, in 2019 the American Society for Transplantation and Cellular Therapy (ASTCT) published recommendations for “an objective, easy-to-apply and accurate classification of CAR T cell-related toxicities,” based on a consensus reached by almost 50 experts in the field (Biol Blood Marrow Transplant 2019, 25:625–38).
Sattva Neelapu, from the Department of Lymphoma/Myeloma, at MD Anderson Cancer Center in Texas, is the senior author of the ASTCT consensus recommendations. He emphasises that CRS and ICANS can be fatal if not recognised. Therefore, patients who undergo CAR T cell therapy need to be managed by specialised teams including physicians with expertise in these toxicities, intensive care specialists and neurologists. Things may be more complicated when the therapy is administered in the outpatient setting. In those cases, patients should be hospitalised as soon as they develop a symptom or sign of toxicity, and caregivers must be taught to recognise symptoms of ICANS.
CAR T cells at a glance
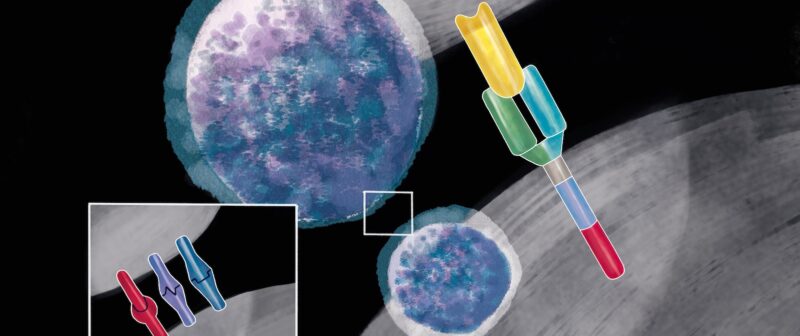
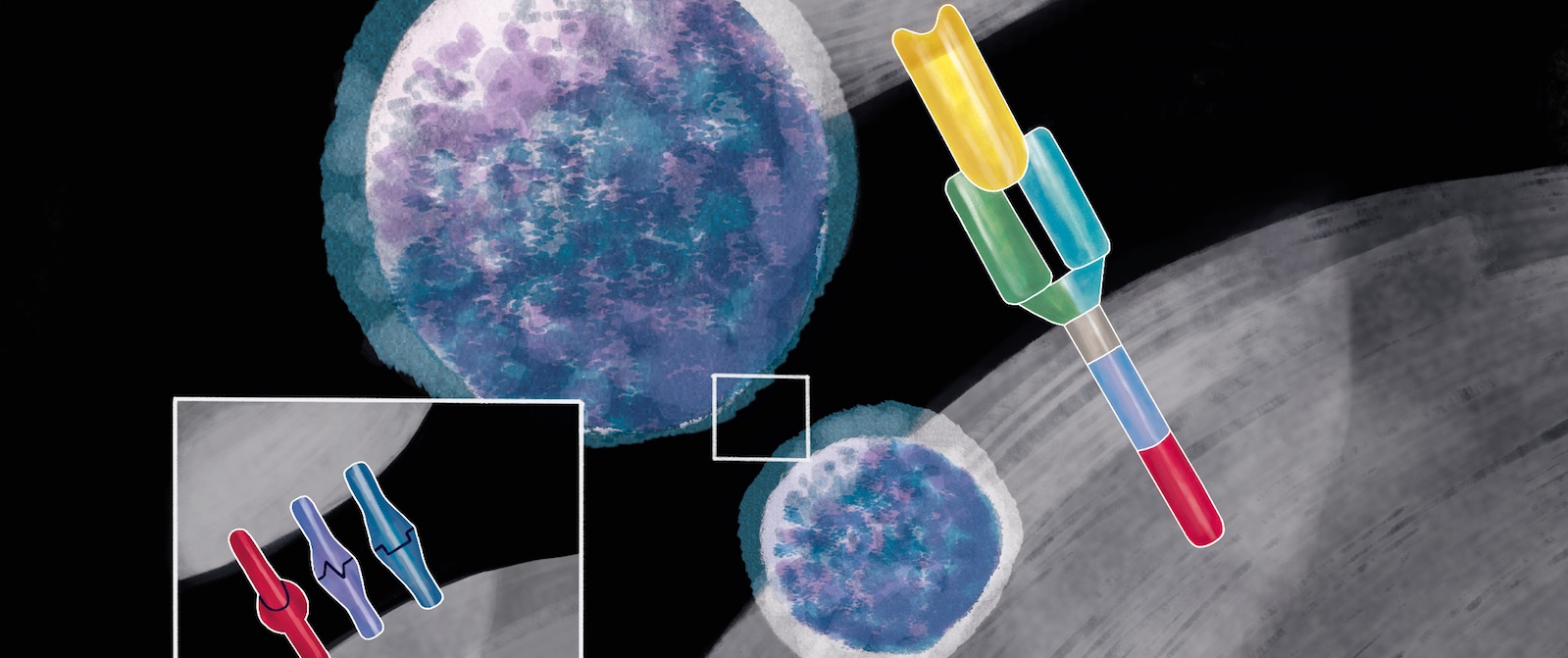
Cytotoxic T cells (CD8) are lymphocytes that can be armed to recognise and destroy cancer cells via the antigens they display on their surfaces. This can be prepared by harvesting a patient’s own T cells from their blood, with a process called apheresis, isolating the cells, and then introducing a chimeric antigen into them, which is done by inserting a gene, mostly using a viral vector, as if ‘infecting’ the cell with the antigen receptor gene. The chimeric antigen receptor (CAR) is a fusion protein. The extracellular portion of the receptor is an antibody-derived targeting domain. It is constituted by the single-chain variable fragment (scFv) of an antibody directed against an antigen expressed by cancer cells. The chimeric construct then enables the cell to express and localise the chimeric antigen receptor to the surface of the T cell, from which location it will be able to recognise a specific marker (known as an antigen) on a cell’s surface. Many different antigens exist on cells, but to date most CARs have been designed to recognise a marker called CD19, which is found on the surface of all B cells (the white blood cells responsible for producing antibodies), including the malignant B cells that cause certain leukaemias and lymphomas. The modified T cells are then cultured and returned to the patient in a single infusion. This is usually preceded by a course of chemotherapy, designed to deplete the patient’s own immune cells, which helps the CAR T cells to multiply in the patient’s body. The CAR T cells then fuse to cancer cells thanks to the CD19 marker, which initiates several signalling pathways, leading to elimination of the targeted cancer cell as well as triggering the ‘expansion’ (multiplication) of the CAR T cells.
Be aware, spot the signs
Cytokine release syndrome
Neelapu outlines some of the signs and symptoms to look out for. “The first clinical manifestation of CAR T cell toxicity is cytokine release syndrome. It usually starts with fever, that can even exceed 40°C,” he says. “Other symptoms are malaise, headache, myalgias, and tachycardia. Possible manifestations include organ dysfunctions, cytopenias, and coagulopathy. In severe cases, patients can develop life-threatening capillary leakage with hypoxia and hypotension.” Rarely, haemophagocytic lymphohistiocytosis – a condition in which the body makes too many activated immune cells – can arise. CRS usually occurs in the first week after CAR T cell infusion, although delayed CRS is possible. Time to resolution is generally seven to eight days, but some patients may need more than 30 days to recover.
Immune effector cell associated neurologic syndrome
Immune effector cell associated neurotoxicity syndrome (ICANS) may occur as a CAR T cell related encephalopathy syndrome.
The clinical manifestations of ICANS are very wide ranging, as toxicity does not affect a specific region of the central nervous system. They include encephalopathy (confusion or delirium), expressive aphasia or language disturbance, motor weakness, myoclonus or tremor, headache, seizures, and a depressed level of consciousness. In rare cases patients can rapidly develop diffuse cerebral oedema. Expressive aphasia seems to be a typical symptom. ICANS onset can range from a few hours to three to four weeks after CAR T cell infusion. It can occur almost simultaneously with CRS or even after CRS has resolved. ICANS is usually self-limiting, and most symptoms reverse in three to four weeks, with persistent abnormalities being uncommon.
Predictors of severe toxic effects
Both treatment-specific and patient-specific factors have a role in determining the gravity of CAR T cell related toxicities, says Neelapu. “The severity of CRS has been correlated with the peak of in vivo CAR T cell proliferation and disease burden. A faster T cell expansion can be promoted by higher cell dose, heavily pretreated bone marrow disease, and also by some kinds of preconditioning, such as fludarabine-containing regimens.” The risk of severe CRS is also increased in patients with comorbidities and in those who develop the syndrome within three days of infusion.
Severe ICANS develops almost only in patients who have experienced CRS, adds Neelapu, with severity being influenced by disease type, disease burden, patient’s age, and treatment history.
Differences in the design of the chimeric antigen receptor may account for variations in toxicity between different CAR T cell products. Second-generation CARs contain an intracellular domain, called co-stimulatory domain, derived from either CD28 or 4-1BB (CD137), to enhance CAR T cell survival and proliferation. CD28-based CAR T cells expand rapidly, while 4-1-BB-based CAR T cells expand more slowly. CRS has an earlier onset with CD28-based CAR T cells, and higher rates of severe neurotoxicity have been observed with CD28-based CAR T cell products. However, an association between the severity of these toxicities and a particular co-stimulatory domain has not been conclusively demonstrated.
The search for biomarkers to predict which patients are likely to develop severe CAR T cell related toxicities, before they become critically ill, is an active field of research.
Pathogenesis of CAR T cell therapy toxicities
The mechanism underlying cytokine release syndrome (CRS) is essentially a ‘cytokine storm’, which produces and sustains a systemic inflammatory response, says Sattva Neelapu, from the Department of Lymphoma/Myeloma, at MD Anderson Cancer Center in Texas. Activated T cells and bystander immune cells, such as monocytes/macrophages and dendritic cells, release several cytokines, including interleukin-6 (IL-6), interferon-γ (IFNγ), granulocyte-macrophage colony-stimulating factor (GM-CSF), and numerous chemokines that recruit more immune cells. The pathogenesis of immune effector cell-associated neurologic syndrome (ICANS), by contrast, is largely unknown, he says. “It is linked with a strong production of cytokines, but none of the cytokines seems specific. Severe ICANS is associated with increased blood-cerebrospinal fluid [CSF] barrier permeability. Elevated levels of cytokines in the CSF may result from both influx and local production.” The accumulation of glutamate and quinolinic acid (two excitatory N-methyl-D-aspartate receptor agonists) in the CSF may explain some of the symptoms, adds Neelapu. The finding that patients with severe CRS and ICANS have high blood levels of angiopoietin-2 also suggests an involvement of endothelial cell activation.
Grading toxicities
For CRS, the ASTCT consensus grading is based on three elements: fever, hypotension, and hypoxia (Biol Blood Marrow Transplant 2019, 25:625–38). Severity can range from grade 1 to 4, with the grade being determined by the most severe event.
The consensus panel that agreed on the recommendations took the decision to focus on criteria that could be measured in the clinic rather than the laboratory, for pragmatic reasons. “Significant alterations in many laboratory parameters clearly occur with CRS,” they wrote. “Cytokine aberrations have been well described, but such data are not routinely available in most academic centres in a time frame that is useful for assigning grade and planning management of a patient experiencing CRS.” They nonetheless encourage clinical teams to monitor cytokines, C-reactive protein, ferritin levels, and other parameters, “so that additional data may be generated for future study”.
For ICANS, the ASCTC consensus grading is based on five elements: the 10-point ICE (immune effector cell-associated encephalopathy) score, depressed level of consciousness, seizure, motor findings, and elevated intracranial pressure/cerebral oedema. Severity can range from grade 1 to grade 4, with the grade determined by the most severe event.
The ICE score is a tool that measures alterations in speech, orientation, handwriting, and concentration. For children aged 12 or younger, the ICE score is replaced by the Cornell Assessment of Pediatric Delirium. “The updated encephalopathy screening tool includes elements for assessing the receptive aphasia seen in these patients,” write the authors of the consensus recommendations, but they add that, while the ICE screening tool is helpful for assessing patients for encephalopathy, the grading of ICANS requires not only assessment of the ICE score but also evaluation of other neurologic domains, as other manifestations can occur with or without encephalopathy.
Making CAR T cell therapies safer
Scientists are working to generate more potent immune effector cells. However, the increase in persistence that would raise CAR T cell anti-tumour efficacy carries the risk of more severe toxicity. From that perspective, a ‘safety switch’ would be highly desirable. Franco Locatelli’s group at the Bambino Gesù paediatric hospital in Rome has developed a 4-1-BB-based CD19-specific CAR construct that incorporates an inducible caspase 9 (iC9) safety switch. The gene of human caspase 9 has been engineered with a drug-binding domain. By administering a nontoxic compound, the iC9 dimerises and activates the cascade domain. In the event of uncontrolled toxicity, CAR T cells can thus be killed by apoptosis within a few hours. Other, more radical, innovative changes include changing cell type. “We are working on trials using CAR-Natural Killer (NK) cells, because this strategy could offer several advantages in comparison to CAR-T cells,” says Locatelli. He sees a number of advantages to this approach. “First, possibly – although it has to be validated in the clinical setting – the NK cell-related toxicity could be lower than that of CAR T cells, because the cytokine production of NK cells has a less toxic, more favourable profile.” Second, the cells could be immediately available, he says, “We can figure out how to prepare banks of CAR-NK cells.” A great advantage is that they can be obtained without the blood apheresis process needed for patients’ T cells enrichment. Third, he concludes, the cancer cell killing effect of the NK cells is greater than that of the T cells, as NK cells are the most potent cytotoxic lymphoid cells in the body.
Managing toxicities
The standard of care for CRS is tocilizumab, an anti-IL-6 receptor antagonist. If the patient does not respond to tocilizumab, corticosteroids can be effective in reversing CRS. Some data suggest that the anti-IL-6 monoclonal antibody siltuximab or the IL-1 antagonist anakinra may have clinical efficacy. In CRS, patients can require vasopressors to correct hypotension and oxygen supply or intubation for hypoxia.
Tocilizumab is not generally recommended for isolated ICANS. In fact, some studies showed a slight increase in severe ICANS rates in patients treated with this antibody. Corticosteroids are widely used to treat ICANS, but type and dose can differ significantly between institutions. Intubation is critical in patients with ICANS who have severely impaired consciousness. Speaking at the 2019 Congress of the European Hematology Association, Stanley Riddell, Scientific Director of the Immunotherapy Integrated Research Center at the Fred Hutchinson Cancer Research Center in Seattle, highlighted the importance of timing and dose in managing CRS.“There are now algorithms to treat CRS with blocking antibodies to cytokines and with steroids, but the timing when you administer those medications can be really critical in determining the patient’s outcome. Being aware of the complications and intervening at the right time and with the appropriate dose of those medications is important,” he said.
Riddell emphasised the urgent need to understand more about the pathogenesis of CRS, to work out and test better ways to manage it. While it is clear that CRS is initiated by the T cells recognising the cancer cells and producing cytokines, he said, “After that there is a cascade of events that is very complicated, involving different cell types.” He pointed to the unexpected finding of several recent studies which showed that, in preclinical models, a major mediator of CRS is adrenaline or its catecholamines. “In the clinic, when patients get CRS, we give them catecholamines to treat their blood pressure, so we are maybe throwing fuel on the fire in some circumstances.”
Riddell anticipates “some major advances” in strategies to avert CRS over the coming year or two. “We need to do the scientific research to understand the pathogenesis and then we need to do the clinical work to test interventions in a logical way on controlled clinical trials, so that we understand which ones are working and which ones do not work,” he said. Right now, it is a manageable problem, he added, “And I am pretty confident that it will be getting increasingly manageable in the future.”