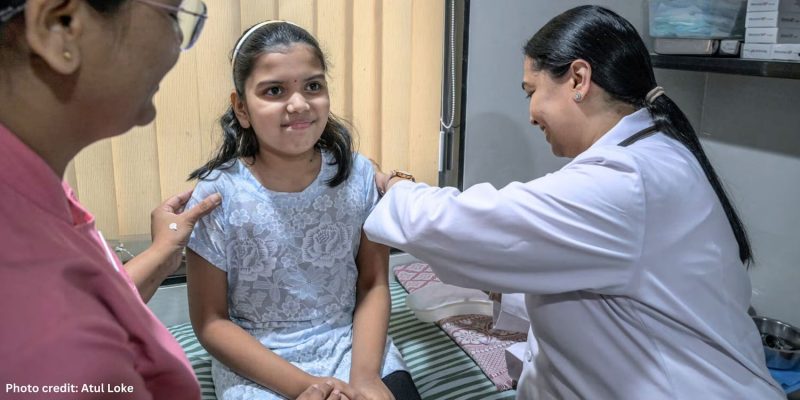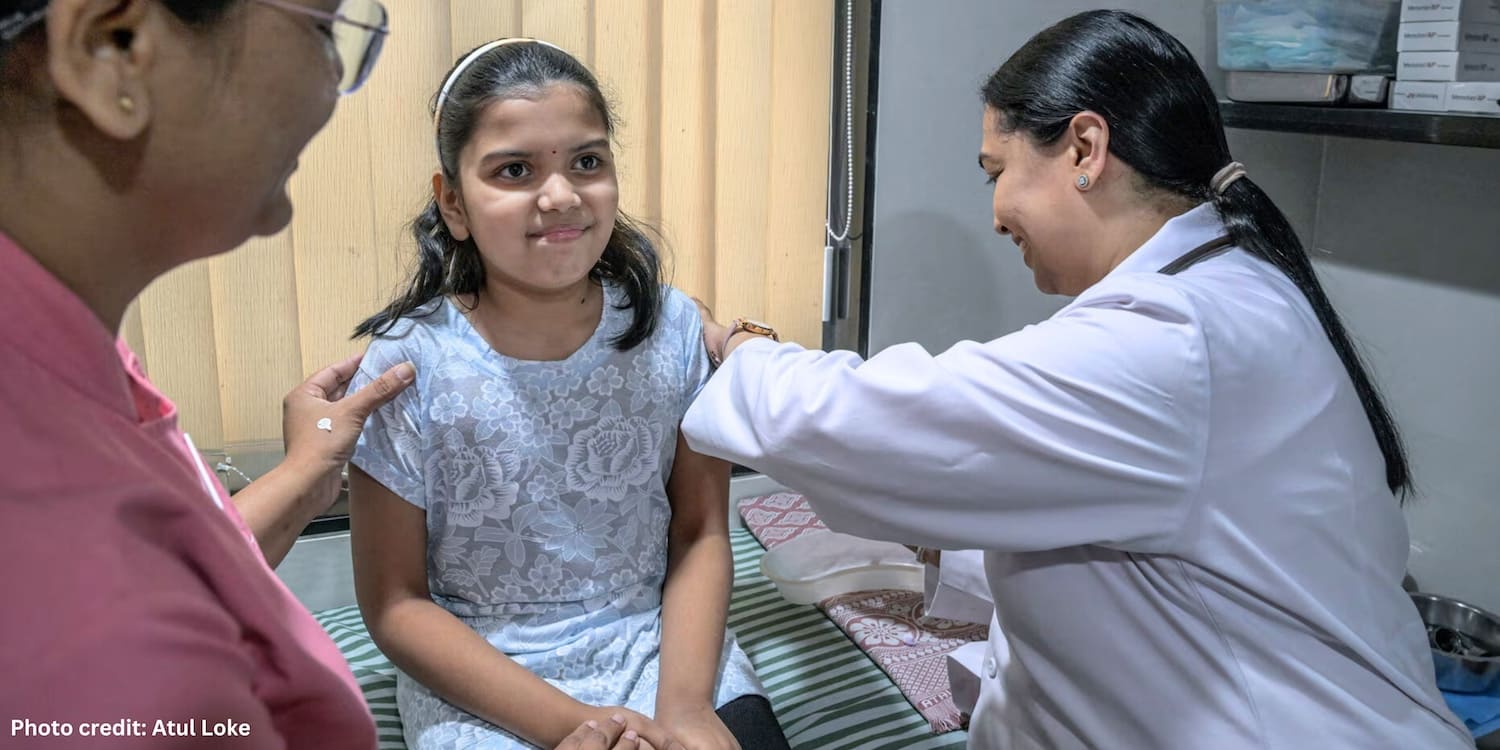It’s hard to remember that, at the time it was introduced in 2008, many questions were raised about whether the human papillomavirus (HPV) vaccine would be cost effective. In the intervening decade and a half, it has become abundantly clear that it is. A 2021 UK study showed that it has reduced cervical cancer rates by almost 90% among women in their 20s who were vaccinated at age 12 or 13. In 2020, the World Health Organization (WHO) announced plans for a ‘cervical cancer-free future’ with the target that, by 2030, 90% of girls in every country would be vaccinated by the age of 15. While many western countries are reaching this target, achieving this goal at a global level will require overcoming cost barriers and supply issues.
The launch of the first HPV vaccine created and manufactured in India could be a huge step forward. Receiving approval from India’s drug controller general in January 2023, the new HPV CERVAVAC vaccine is the result of a partnership between the Serum Institute of India (SII), based in Pune, India’s Department of Biotechnology, the Biotechnology Industry Research Assistance Council, and the Bill & Melinda Gates Foundation. The quadrivalent vaccine is effective against four HPV types – 6, 11, 16 and 18, with 16 and 18 being the oncogenic strains responsible for 70% of cervical cancers, and 6 and 11 responsible for genital warts, which can also be hard to treat.
The Indian government is now planning a comprehensive country-wide vaccination programme, with the eventual aim of providing protection against cervical cancer for India’s 700 million women and girls. This comes 16 years after the first disastrous attempt at national coverage in 2008, which used the then newly developed HPV vaccines from Merck and GSK. “Early programmes were associated with deaths that were completely unrelated to the vaccination… but they led to a 10-year ban,” says Paul Bloem, Senior Technical Officer and HPV Vaccine Impact Lead at the WHO in Geneva.
The scare about safety issues erupted in 2010, when an Indian Parliamentary Standing Committee on Health began probing the use of HPV vaccines in two states, after the reported deaths of seven girls. The committee found that the “safety and rights of children were highly compromised and violated”. The finding related purely to regulatory and ethical issues around whether the vaccinations should never have taken place outside of a registered clinical trial, with all the safeguards and safety reporting that would entail. That is not how it was perceived by the media and wider public. And while clinicians argued that the report ignored extensive evidence about the safety and efficacy of HPV vaccines, and further state investigations of the deaths – and the enquiry committee itself – concluded that there was no evidence that any deaths were caused by the vaccines, the damage had already been done. “It derailed the process,” says Ishu Kataria, a Senior Public Health Researcher with the RTI Center for Noncommunicable Diseases, a non-profit research institute based in Delhi, “[and it] took many years for the momentum to come back.”
Without a national vaccination programme, cervical cancer remains a huge health burden, with around a quarter of global cervical cancer cases and deaths estimated to occur in India. “It’s the third most common cancer in the country, women’s second most common killer, and one death every seven minutes in the country is due to cervical cancer,” says Kataria.
The SII are currently manufacturing 70 million doses a year, and expect to double this by 2026
The hope is that the new indigenous vaccine will provide momentum for change. The clinical trials have shown CERVAVAC to be non-inferior to the quadrivalent Gardasil vaccine from Merck, in girls and boys aged 9 to 14, with comparable immune responses and safety profile. The SII are currently manufacturing 70 million doses a year, and expect to double this by 2026.
Kataria says that, although things have been on hold during the Indian election (which concluded on 1 June 2024), the government has now allocated a budget for a national HPV vaccination programme, which she calls the ‘missing piece of the puzzle’. “This is something that we should have done before as a country. But I’m glad it has been done.”
Good news for global costs and access
CERVAVAC is not the only recent entry to the HPV vaccines market. In addition to the original Cervarix from British pharmaceutical major GSK and Gardasil from Merck, in October 2021, the WHO pre-qualified Cecolin, China’s first self-developed HPV vaccine against HPV 16 and HPV 18, which means the WHO has assessed the quality, safety, and efficacy of the vaccine for international procurement to developing countries. Cecolin is about two-thirds of the price of the other vaccines, but the hope is that the Indian vaccine, which is expected to be available globally by 2026, will reduce prices even further. “We don’t have a formal price,” says WHO’s Bloem, “[so] how much lower is speculation for now.”
Kataria expects the price for low-income countries will be less than half the price of Gardasil, and the competition is likely to lower prices overall. “This is probably a game changer,” she says. Bloem anticipates that the impact may even be greater for middle-income countries, “because they are the ones who pick up all the costs.” The poorest countries usually receive free vaccines from Gavi, the Vaccine Alliance, the public–private global health partnership (previously the Global Alliance for Vaccines and Immunization).
Kataria expects the price for low-income countries will be less than half the price of Gardasil, and the competition is likely to lower prices overall
The new vaccine will also address recent supply issues resulting from the current duopoly, which are derailing some global vaccination campaigns. Bloem mentions a recent production problem that left a shortfall of 10 million doses. “We’re very vulnerable to sudden upsets, so having a big producer that is able to produce millions of doses will help.” Supply problems have also been an issue in the small number of Indian states that have set up their own programmes, says Kataria, “the SII vaccine will be able to curb that supply issue…[and] if they can do it here, they can also challenge the monopoly that is set by the two players that currently supply the vaccine.”
Single dose approach to expand coverage
The new HPV vaccine will be used in the national programme which intends to initially vaccinate girls between nine and 14 years with two doses of the vaccine given six months apart. But the plan is to change to a single dose, in line with revised international recommendations, and extend the vaccine to boys as well, says Kataria.
Since 2023 the WHO has been advising that one dose is sufficient for 9- to 14-year-olds, with two doses for those aged 15 to 26 (for whom the previous recommendation was for three doses within six months). The revised recommendations are based on a study carried out by the International Agency for Research on Cancer, involving 15,000 girls who received 1, 2 or 3 doses, who underwent immunological testing over a 10-year follow-up.
The study concluded that one dose of HPV vaccine provides similar protection to that provided by two or three doses against persistent infection with HPV16 and HPV18. While no statistical differences were found between the impacts of the regimens, the trials were not set up to show non-inferiority, and Bloem says it will take a further year of follow-up to get a definitive answer.
Many countries have already switched to one dose, including the UK and countries receiving their vaccines via Gavi. The cost savings from needing fewer doses of the vaccine per child, as well as from lower administration costs, will be significant. Even if the single dose were to offer slightly inferior protection, modelling of costs in India shows that, over 10 years, a single ‘catch-up’ dose for all girls and women aged 11 to 20 years would be more impactful than starting a two-dose vaccination in the cohort of girls age 10, leading to an estimated 39–65% versus 38% reduction of lifetime cervical cancer risk in those cohorts.
Hitting the 90% target
With progress in tackling costs and simplifying administration, the focus remains on tackling challenges around capacity and building confidence and demand at a community level. HPV vaccination programmes took a huge hit during the Covid pandemic. “We [saw] a 25% drop [in HPV vaccinations] relative to where we were,’ says Bloem. Global vaccination rates are now starting to recover, he adds, although this is partly due to some countries launching new vaccination programmes, with uptake levels in many countries still down on pre-Covid levels. “This year, we expect better recovery, but it’s still too early [to tell].”
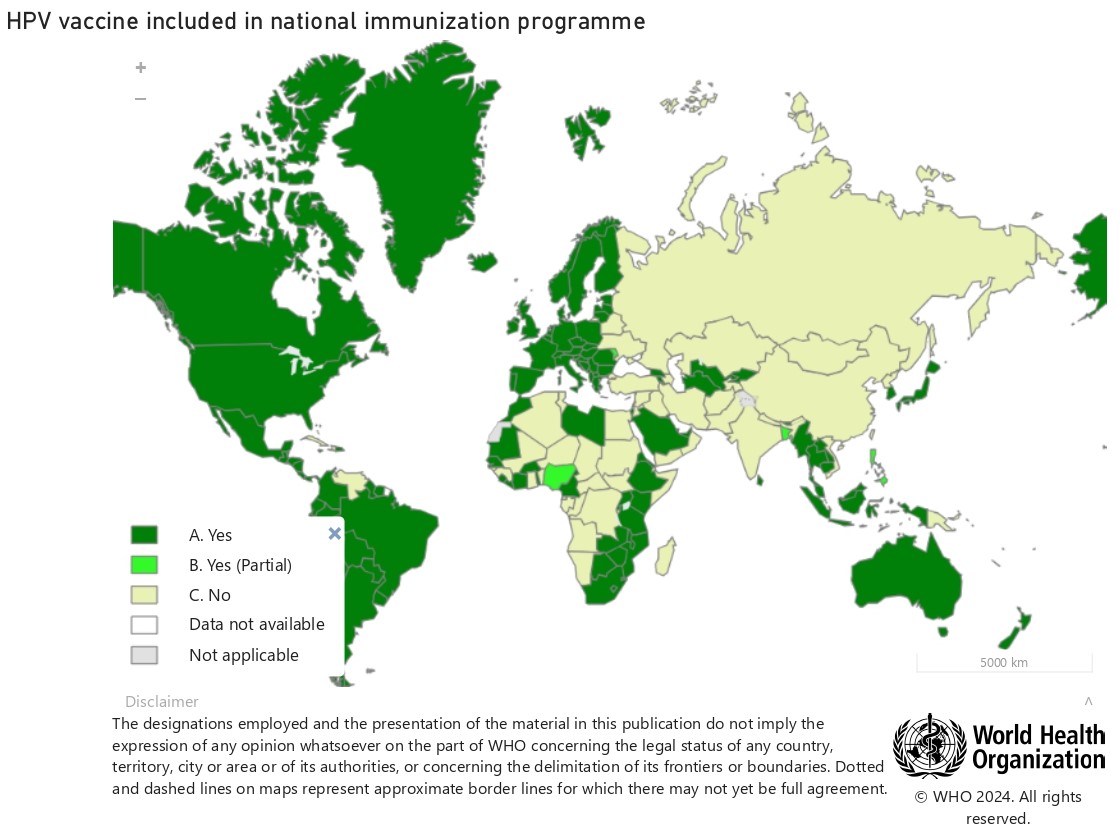
Source: World Health Organization © WHO 2024
In Africa, fears that spread in relation to Covid vaccines, and some related public pushback, seems to be impacting uptake of the HPV vaccination, says Bloem. “We still get questions sometimes, ‘Is this not the Covid vaccine that you’re trying to send out?’” he says. He adds, however, that the pandemic didn’t just fuel vaccine hesitancy, it also convinced a lot of people about the tremendous benefits vaccines can offer, which may be pushing up HPV vaccination levels in some countries.
In India, the profile of the Serum Institute of India, which is known for making indigenous vaccines that are widely used in many low- and middle-income countries, is likely to have benefited immensely from its role during Covid, where it partnered with AstraZeneca to manufacture its Covid vaccine under licence.
Happily, says Kataria, India is not a very vaccine hesitant country, but engaging with communities will still be essential for success: “The communication campaign is going to be the key thing.” This will have to include information about the vaccine and about cervical cancer, and debunking of any myths and misconceptions. Vaccination programmes have already been rolled out in the states of Punjab, Delhi and Sikkim. Each uses slightly different delivery methods, but they all carried out extensive communication campaigns for six months before the vaccine appeared in any school or health facility. The messaging was targeted at both girls and their parents, as well as at school authorities and local health workers and officials, who are trusted in these communities. “They also included the media,” adds Kataria, “which I thought was a pretty good thing, because it was the media many years back that had painted a picture that this vaccine is going to kill people.”
“Across India as a whole, public understanding of cervical cancer and the benefit of vaccination remains very low”
Having spoken with those involved, Kataria is confident that the communications campaigns were effective. One of the big worries in conservative areas centred around whether the vaccine could promote sexual promiscuity. “They were able to debunk those myths, using a lot of the senior leaders from the health department to go and address concerns – it took them a solid six months before people were convinced,” she says. Across India as a whole, however, public understanding of cervical cancer and the benefit of vaccination remains very low, she stresses. “People just don’t know about it, including people who are very highly educated that I’ve spoken to, who seem very surprised to know that there is a vaccine that can actually prevent cancer of the cervix… it’s not only the rural populations, or people who’ve not been educated.”
Getting the implementation right will also be crucial says Bloem. Centrally planned systems do well in their coverage levels, but also countries who have effective management. “We have a couple of countries where they have reached 90% for a long time, including Uzbekistan and Turkmenistan. Ethiopia is a great example of a country that does very well,” he says. In most cases the key seems to be commitment and confidence at the top. Conversely, if the leadership seems afraid or hesitant in the early stages, he warns, that will also be contagious. “We’ve seen this in Japan,’ he says, where the Ministry of Health temporarily suspended vaccinations in 2013, after reports of adverse reactions, only ending the suspension in 2021, but vaccination rates remain very low.
He points to the current situation in India as a positive example of how public confidence can be regained, “You can see how things can shift and how the… confidence of the leadership and the Ministers of Health in the vaccine, to stand behind it, can be an important factor.”
Good news on herd immunity
While the WHO has set a target of 90% coverage, the good news is that research seems to be showing that, even at vaccination levels of only 50%, there are herd immunity effects that could be protecting a wider population (because enough people have developed immunity to severely reduce the levels of HPV infection in circulation). A 2015 analysis of 20 eligible studies – all done in high-income countries – representing more than 140 million person-years of follow-up, showed that, where female vaccination coverage was at least 50%, not only did HPV type 16 and 18 infections decrease by 68% and anogenital warts by 61% among girls aged 13 to 19, but there was also a strong suggestion of a herd immunity impact, with significant reductions in anogenital warts reported also in boys younger than 20 years of age and in women aged 20 to 39.
By contrast, in countries with female vaccination coverage lower than 50%, while significant reductions in HPV types 16 and 18 infection and in anogenital warts occurred in girls younger than 20 years of age, there was no indication of herd immunity. “We have no particular number to say herd effects occur after a certain percentage coverage,” says Bloem, “[but] there does seem to be somewhere an inflection point… There is a lot of talk of HPV 16 and 18 virtually disappearing from the landscape in places such as the UK, where you reach such high coverage among these populations that have been vaccinated.”
Currently, nine in ten deaths from cervical cancer occur in low- and middle-income countries. Although 142 countries have implemented vaccination programmes, only 21% of girls in the target age group have had at least one vaccine. India and China’s entry into the HPV vaccine market should help to improve the feasibility of rapidly increasing that vaccination rate, by addressing cost and supply issues. “It will take a lot of work,” says Bloem, “but it can be done.”
The opening image shows Dr Sunita Lalwani administering the CERVAVAC vaccine, which will be offered free to all girls between the ages of nine and 14.
Photo credit: Atul Loke. Published here by kind permission. Atul Loke ©2024