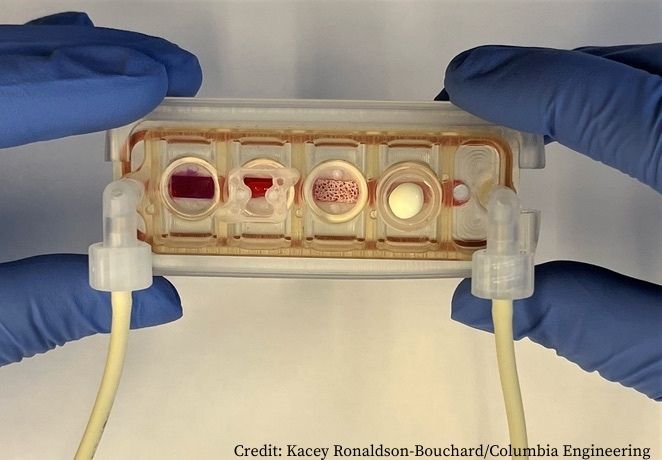
A ‘plug-and-play’ multi-organ chip, the size of a microscope slide, could be customised for individual cancer patients to determine personalised therapy. The study, published in Nature Biomedical Engineering, reports for the first time the achievement of connecting a range of millimetre-sized engineered tissues (beating heart muscle, metabolising liver, and functioning skin and bone) linked by recirculating vascular flow, allowing for independent organ function.
“We are excited about the potential of this approach. It’s uniquely designed for studies of systemic conditions associated with injury of disease, and will enable us to maintain the biological properties of engineered human tissues along with their communication. One patient at a time, from inflammation to cancer!” says Gordana Vunjak-Novakovic, the project leader from Columbia University School of Engineering and Applied Science, New York. “This is a huge achievement for us – we’ve spent ten years running hundreds of experiments, exploring innumerable great ideas, and building many prototypes.”
To enable in vitro modelling of human physiology, microphysiological systems are being designed using bioengineered human tissues to mimic organ-level functions. One obstacle has proved to be that tissues cultured in isolation fail to take into consideration systemic interactions influencing organ responses to injury, disease and therapy. Additionally, use of common media can induce committed cells to revert back to more plastic and immature phenotypes. The result has been that disease impacting several organs and vascular flow have proved challenging to emulate in vitro.
In the study, Vunjak-Novakovic and colleagues report on the design of a ‘multi-organ’ tissue chip allowing integration of bioengineered tissues by providing each tissue with its own specialised environment, maintained by a selectively permeable endothelial membrane linked to other tissue compartments. Key features of the system include:
- Tissues were engineered from human-induced pluripotent stem cells for biological specificity and combined with supporting stromal cells within an extracellular matrix, and matured individually for four to six weeks under conditions promoting their phenotypes.
- Each tissue was cultured in its own optimised environment and separated from the common vascular flow by a selectively permeable endothelial barrier.
- Beneath each tissue chamber was an inert elastic mesh covered with endothelial cells and supporting mesenchymal stem cells forming a vascular barrier with 20 μm pores used to support formation of confluent endothelium. Tissues were able to communicate with each other through cytokines, exosomes and cells in the circulating flow below the vascular bed.
- The tissue chip was manufactured from polysulfone, a biocompatible inert polymer, with modular design allowing tissue to be placed in a well after it has passed quality control.
- Each tissue on the chip (heart, bone, liver and skin) was selected for having distinctly different properties and roles in modelling diseases and testing drugs.
- The tissue chip uses a single channel of a peristaltic pump to recirculate culture media at set flow rates and shear stresses.
To validate utility of the multi-organ tissue chip, the team evaluated maintenance of tissue phenotypes over four weeks of culture. Maintenance of matured tissues in the multi-organ tissue chip was compared to that of a mixed tissue chip (equivalent to the multi-organ tissue chip except for the lack of endothelial barrier, thus representing the common medium culture) and isolated cultures (tissues cultured individually, with and without endothelial barriers, representing the gold standard). To explore the capability of the chip for elucidating the pharmacokinetics and pharmacodynamics of therapeutic agents, the team modelled the cardiotoxicity of doxorubicin, a drug used in treatment of several types of cancer.
Results showed that:
- Over four weeks of culture all tissues in the multi-organ tissue chip maintained the structural, functional and molecular stability of the ‘gold standard’ isolated group, markedly exceeding tissue properties of the mixed group.
- Time-concentration profiles of doxorubicin and doxorubicinol (liver metabolite of doxorubicin) in the multi-organ chip closely matched measurements taken in human subjects.
- Early miRNA biomarkers of cardiotoxicity following exposure to doxorubicin showed a statistically significant similarity with clinical data in the multi-organ chip (P=0.0021), which was not shown in the mixed tissue chip (P=0.11). The clinical data used in the comparison was suggested by a recent clinical study identifying 17 miRNAs differentially expressed in paediatric cancer patients who developed left ventricle failure following treatment with doxorubicin.
“Vascularly linked and phenotypically stable matured human tissues may facilitate the clinical applicability of tissue chips,” conclude the authors. “This study suggests that the multi-organ chip can serve as a patient-specific model for developmental testing of new therapeutic regimens and biomarkers of drug toxicity, on the basis of its ability to maintain the biological fidelity of each tissue while also allowing their communication.”
Medium separation, they add, is particularly important for tissues derived from human-induced pluripotent stem cells, which maintain some developmental plasticity and are not fully matured.
“The endothelial barrier between the tissues and vascular flow promotes physiological cell and tissue responses due to the paracrine signalling, selective transport of drugs and secreted factors , and immune cell extravasation,” write the authors.