CRISPR is a revolutionary gene-editing tool that allows scientists to cut DNA with extraordinary precision and make changes to the genome. The technology was a gift to cancer researchers, whose efforts to understand the roles played by different genes rely on seeing what happens when a gene is knocked out or modified.
For centuries this work was limited to studying the effects of spontaneous mutations, or using substances that introduced random errors into the genomes of plants, animals or cell cultures. New techniques for gene editing developed over recent years allowed more specific modifications to be introduced to the genes under study, greatly speeding up progress in biology research. But the first techniques for carrying out ‘cut and paste’ operations on DNA fragments, by modifying their sequence, had low specificity and were quite expensive and time-consuming.
The announcement of the CRISPR/Cas9 ‘genetic scissor’ in 2012 was a game changer, which earned the lead scientists, microbiologist Emmanuelle Charpentier and biochemist Jennifer A. Doudna, the 2020 Nobel Prize in Chemistry.
CRISPR technology caused a major stir in the field of genome editing and became popular as a research tool, because it makes genome editing much simpler and faster. Researchers now use CRISPR as the default technique to manipulate the genome in many applications, including functional genomics, DNA imaging, diagnostics and drug development. This technology is also being investigated as a potential treatment for a wide range of diseases.
In cancer, CRISPR is significantly improving our understanding of the biology of the disease, helping researchers investigate the activities of cancer-related genes, generate animal models of cancer, and probe drug targets. Recently, CRISPR technology has also started to be used in experimental cancer treatment, and it is promising to have an impact in cancer therapies in the coming years.
A genetic scissor
Science is about serendipity, and CRISPR – like many other advances in science and medicine – was brought to light by researchers who stumbled across it when they were looking for something completely different. In this case, the research was into how microbes fight viral infections. Scientists studying this topic found that, to protect themselves against invaders like viruses, bacteria capture small pieces of the virus’s DNA, which they store within their own chromosomes. These fragments, identified by a set of DNA segments called CRISPRs (clustered regulatory interspaced short palindromic repeats), act as a record of past infections, and convey immunity to future infections. What happens when the same virus attacks the bacterium again holds the key to CRISPR gene editing technology.
When the bacterium detects the presence of virus DNA that it has encountered previously, the DNA fragments (CRISPRs) stored in their chromosomes are translated into two short RNAs, one of which has a sequence that matches the one of the invading viruses. These two RNAs combine with a protein called Cas9. Cas9 is a nuclease, an enzyme capable of cutting DNA. When the matching RNA sequence finds its target within the viral genome, the Cas9 cuts the target DNA, inactivating the virus.
When this immunity system was discovered, scientists realised that it could be exploited to cut any DNA sequence at a selected site simply by modifying the ‘guide RNA’ to match the target DNA.
When this immunity system was discovered, scientists realised that it could be exploited to cut any DNA sequence at a selected site
In the laboratory setting, the CRISPR gene-editing tool is made up of a guide RNA (gRNA) and a DNA-cutting enzyme, usually Cas9. Researchers model the gRNA to bind to the DNA of the gene to be modified (called the target). The gRNA associates with Cas9 and steers Cas9 to the target. Once inside the nucleus, Cas9 recognises a sequence known as PAM – a short DNA sequence, the protospacer-adjacent motif, used to mark a target site – and unzips the DNA, allowing the matching with gRNA. If the match is complete, Cas9 cuts the DNA. Once this happens, the cell tries to repair the cut, but this process is defective and can generate mutations that disable the gene. These mutations are random, but researchers can also make ad-hoc changes, such as targeted deletions and precise gene replacement. This can be done by adding another piece of DNA with the new sequence to be added. Once the CRISPR system has made the cut, the customised DNA pairs up with the cut ends, recombines and replaces the original sequence with the new version.
The introduction of CRISPR transformed the gene editing process. Previous tools, such as ZFN (zinc-finger nucleases) and TALEN (transcription activator-like effector nucleases), were costly and complex. Each edit required the creation of a specific ZFN or TALEN protein, and engineering proteins is a difficult, time-consuming and expensive process. With CRISPR, researchers just need to create a short RNA template that matches the target DNA sequences; it’s a much faster, simpler and cheaper process than engineering proteins.
CRISPR’s many uses in cancer research
Mapping cancer genes
“If you know the enemy and know yourself, you need not fear the result of a hundred battles.” This famous quote from military strategist Sun Tzu applies equally to oncology research, where discovering new molecular targets for the next generation of drugs starts with identifying the gene associated with tumour development and finding the weakness of cancer cells.
CRISPR technology has revolutionised genomics and dramatically increased the efficiency of functional genetic screens, where hundreds of genes must be independently deleted to identify key regulatory proteins and test new potential drugs. The CRISPR gene-editing tool can be easily used to cut genes known to be associated with tumour growth and metastasis, to identify the ones essential to the process. This same technique can also be exploited to track and identify gene interactions.
CRISPR can be easily used to cut genes associated with tumour growth and metastasis, to identify the ones essential to the process
In a pivotal 2019 paper published in Nature, a research group from the Wellcome Sanger Institute located just outside Cambridge, UK, used CRISPR to develop a ‘molecular map’ of cancer, which identified 600 possible targets for new drugs. In this study, the researchers knocked out genes in more than 300 human cancer cell lines from 30 cancer types and developed a data-driven framework to identify new candidates for cancer drugs. By using CRISPR, researchers were able to cut portions of DNA with great precision, and turned off hundreds of genes in cells from different types of tumours. The aim of the experiment was to evaluate the impact of the deleted genes on the survival and replication of the tumour itself.
The tumour cells with genes that had been knocked out were cultivated for two weeks. If the cells showed difficulty in growth and proliferation, the deleted gene was identified as crucial for the survival and proliferation of the cells and could be a potential target for a successful anti-cancer strategy. After a deep bioinformatics analysis, which aimed to exclude genes found to be essential to healthy cells as well as tumour cells, the researchers identified 600 possible new targets for future drugs to aim at. With this method, researchers were also able to put target-identified genes into a priority list, ranking them from the most promising to the least, to narrow down the number of candidates.
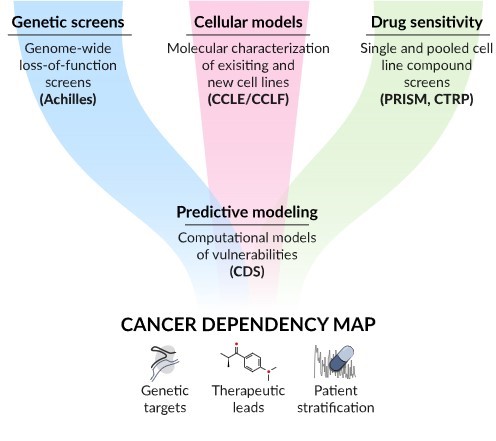
The CRISPR gene-editing tool is one of the pillars of the Cancer Dependency Map project, a very ambitious project, whose goal is to map all cancer dependencies and identify all genes that are essential for the survival of cancer, which could become the target of specific drugs. The project, which is supported by the strategic collaboration of two of the most important genomic research institutes in the world: the Wellcome Sanger Institute and the Broad Institute of MIT and Harvard (Cambridge, Massachusetts), “aims to assign a dependency to every cancer cell in a patient which could be exploited to develop new therapies”.
Mapping cancers’ vulnerabilities
Individual cancers rely on distinct essential genes for their survival. For most cancers, the relationship between the genetic alterations and the dependencies they cause is not fully known. The Cancer Dependency Map is an ongoing project to identify and systematically map gene alterations by intervening on each gene and protein and assessing what happens in cancer cells as a result of these perturbations, in different cancer models, at different stages of disease development, and in different clinical settings.
The Cancer Dependency Map project was born as a strategic collaboration between the Wellcome Sanger Institute (near Cambridge, UK) and the Broad Institute (Cambridge, Massachusetts) and is committed to open science.
For more information see DepMap: The Cancer Dependency Map Project at Broad Institute
Image source: DepMap Portal ©Broad Institute
Generating cancer models
Since its inception, CRISPR has been used to generate in vitro and in vivo models to study the role of specific genes in cancer initiation and progression, providing insight into how to prevent or treat several types of cancer.
In cell culture, scientists use CRISPR to understand how the inactivation of certain genes, commonly mutated in a specific cancer, contributes to tumour progression. The CRISPR editing tool can be used to delete genes of interest in cancer cell lines and study the survival of these cells and their sensitivity to drug treatment, revealing mechanisms of cancer development and potential therapeutic target.
This approach has significantly improved our understanding of the role and activities of oncogenes and tumour suppressor genes in cancer. Oncogenes are mutated genes that can promote the transformation of normal cells into cancerous ones. In several studies, the CRISPR system was used to delete and/or modify the activity of oncogenes and evaluate the therapeutic potential of oncogene targeting. CRISPR has also been used to study tumour suppressor genes. Inactivation of tumour suppressor genes is a crucial step in the initiation and progression of cancer and the application of the CRISPR system has led to rapid identification and validation of several tumour-suppressor genes.
Researchers have applied the CRISPR system to develop multiple animal tumour models, which are used to understand the development and progression of individual tumours and identify new strategies for cancer treatment. In gene knockout animal tumour models, ‘genetically engineered’ animal models, gene editing tools are used to delete genes of interest and to study the role of a single gene or several genes in tumourigenesis.
Nowadays CRISPR technology is totally integrated into cancer cell molecular biology research, allowing the generation of precision tumour animal models that promote comprehensive research on tumour development and cancer-related genes, and pave the way for precision cancer medicine.
Using CRISPR to treat cancer
Will CRISPR find a role in treatment strategies? Studies using CRISPR in a therapeutic role in patients are currently at a very early stage. The first clinical trial to investigate a CRISPR-based cancer therapy was launched in 2018 by Edward Stadtmauer and colleagues at the University of Pennsylvania Abramson Cancer Center in Philadelphia. The phase I trial, partially funded by the US National Cancer Institute, aimed to assess the safety and toxicity of a type of immunotherapy in which patients’ own immune cells were genetically modified, using CRISPR, to better ‘see’ and kill their cancer. Specifically, it aimed to improve the function and persistence of engineered T cells used in adoptive T cell therapies such as CAR T cell therapy.
Three patients were enrolled in the study: two women with advanced multiple myeloma, aged 62 and 67 years respectively, and a man with metastatic sarcoma aged 65 years. All three had previously received chemo and radiotherapy and had no remaining treatment options.
In Stadtmauer’s trial, researchers withdrew T lymphocytes from the patient’s blood and used CRISPR-Cas9 to disrupt three genes: two that interfere with the activity of the receptor and one, PD-1, which produces a protein that limits the cells’ cancer-killing abilities and is one of the key targets for immune checkpoint inhibitors. In addition, they added a cancer-targeting transgene, NY-ESO-1, which improves the ability of T cells to recognise and fight the cancer. The engineered T cells were then multiplied in vitro and re-infused into patients.
Modifying T cells can be a lengthy process, with most CAR T cell therapies using chimeric antigen receptors individualised to antigens isolated from the patient’s own tumour cells. The use of CRISPR to remove genes that inhibit the cells from working and make them more aggressive – together with the addition of a generic anti-cancer gene that armed the T cells against an antigen found across a variety of tumours – made the gene editing process cheaper, quicker and more efficient.
Initial findings published in Science suggested that the treatment is safe. Six months after the treatment, the three patients experienced mild side effects, most likely caused by the chemotherapy received before, and no toxicity or signs of immune reaction to the CRISPR-edited cells.
Unfortunately, the treatment had only a minor impact on the tumours of the three patients. In two of them (one with sarcoma and one with multiple myeloma) the cancer stopped growing for a while, but later started spreading. The treatment had no benefit for the third patient, who died.
Another T-cell therapy that recently took advantage of CRISPR is engineered T cell receptor (TCR) therapy. Like CAR T cell therapy, engineered TCR therapy consists of treating cancer with activated T lymphocytes from the patient. While CAR-T cell therapy targets antigens expressed on the surface of cancer cells, in the engineered TCR therapy, the added receptors are addressed against antigens expressed inside tumour cells. The advantage of this therapy is that engineered T cells can recognise and target intracellular proteins essential for the survival of cancer cells and can be used to treat different types of cancers, including solid tumours.
In a study published in Science Translational Medicine, Bonini and her team at the San Raffaele Institute in Milan, in collaboration with the American biotech company Intellia Therapeutics, replaced receptors normally expressed on T cells with T receptors isolated from the blood cells of healthy donors and able to recognise WT1 – a specific tumour protein. WT1 is a transcription factor involved in cell growth and differentiation, whose expression is strongly associated with oncogenesis. Researchers used CRISPR technology to knock WT1-specific T receptors into patient T cells and generate an autologous cell therapy.
Researchers replaced receptors normally expressed on T cells with receptors from healthy donors, able to recognise WT1
The result was an army of highly tumour-specific T lymphocytes. Based on the safety and efficacy results obtained in preclinical models, in early 2022 regulatory bodies in the US and UK gave the green light to start the first clinical trial in patients with acute myeloid leukaemia resistant to current treatments.
Another important clinical study using CRISPR to treat cancer was recently described in a paper published in Nature. The trial involves 16 patients with solid tumours affecting various tissues and organs, including breast and colon, who had relapsed following standard treatment for their cancer. Researchers used CRISPR technology to personalise the therapy and treat patients with T cell receptors that had been genetically modified to specifically attack their individual cancer.
Susan Foy and her group began by sequencing the DNA of each subject’s blood cells and tumours to identify the mutations present only in tumour cells and not in healthy tissue. Once mutations were identified, they used an algorithm to identify the mutations able to stimulate the best immune reaction from T cells. Using this information, researchers selected immune cells whose T cell receptors matched the cancer mutations and expanded this population of cells in vitro. To further improve T cell performance, researchers used the CRISPR gene-editing tool to insert a gene that equipped T cells with receptors that enable them to recognise the specific mutations of the single tumour. Before re-infusing engineered CAR T cells, the researchers treated the patients with chemotherapy in order to reduce the existing immune cells.
The study showed that modified CAR T cells were able to quickly expand and became the dominant immune cell population, often representing more than 20% of the white blood cells clustered near the tumour they were designed to target. One month after treatment, patients remained stable (they did not worsen) and only two reported side effects. These efficacy results might seem rather modest, but the researchers used low doses of engineered CAR T cells to assess the safety of the treatment. The next step will be to use higher doses and engineered CAR T cells to both recognise tumour mutations, and also escape from the immunosuppressive signals released by solid tumours.
Risks and limitations
While CRISPR technology has been a game-changer for cancer research, this gene editing tool has several limitations that must be addressed, particularly in therapeutic settings.
Key among them is that CRISPR may cut DNA outside of the target gene, an effect known as ‘off-target’ editing. These unintended edits could cause unwanted gene disruption or large-scale chromosome aberrations that could impair normal cell function, potentially turning normal cells into cancerous ones. In laboratory settings, such issues can be minimised by using ad-hoc control techniques. It is important to bear in mind, however, that that any genome manipulation may have important consequences that in turn may affect the use of CRISPR-based strategies, particularly in clinical use.
Delivering CRISPR components into cells is another potential limitation to the large-scale implementation of this technique. In a laboratory setting, delivery of CRISPR into the cells is achieved through viruses whose genome is modified with the insertion of the genes for the guide RNA and Cas9. This approach may be challenging in vivo, however, as viruses that carry CRISPR may target multiple cell types, and this could result, for instance, in the editing of lung cells, when the goal was to edit liver cells.
To overcome this lack of specificity, researchers are investigating alternative ways to deliver CRISPR. One approach may be to use viruses that target only a specific organ or cell type. Alternatively, researchers are also investigating the use of nanocapsules, polymer-based structures widely studied as potential drug delivery systems.
Another potential obstacle to the use of CRISPR as a gene-editing tool in vivo is that manipulating cells inside the body could affect germline cells and thus be transmitted to future generations. In the case of current clinical trials involving CRISPR, the gene editing is carried out outside the patient, on cells removed from their body. This ‘ex vivo’ approach is deemed more secure and controlled than editing cells inside the body.
Finally, affordability of CRISPR therapies may become a major issue – this was one of the main topics discussed at the Third International Summit on Human Genome Editing held in London in March 2023. The final document released by the members of the organising committee noted that, as therapies based on CRISPR become more widespread, “a commitment to equitable, financially sustainable, and accessible treatments becomes more urgent.” It recommended that, “health care systems and the global health community should prepare to provide patients with cost-effective, affordable, proven therapies.”
Looking ahead
Scientists see myriad ways that CRISPR could advance cancer research, and with the continuous improvement of gene editing tools and the identification of new effective targets for diseases, the clinical translation and application research of gene editing technology will further expand.
The development of CRISPR gene editing technology will further encourage therapeutic applications and offer a broad range of therapeutic strategies, especially for malignant tumours.
In the last ten years, the use of CRISPR gene editing technology as a successful therapeutic strategy has been explored in several preclinical and clinical studies. However, the clinical trials conducted until now involved a small number of patients with a limited follow-up. Further in-depth in vivo research studies must be planned.
“It’s tough to make predictions, especially about the future,” as the saying goes, but CRISPR is here to stay, and over the next 5–10 years CRISPR will take its first real steps into clinical medicine.