Messenger RNA vaccines turned around Europe’s fight against the Covid pandemic. Less than a year after the first lockdowns were declared, mRNA vaccines got regulatory approval for emergency use, first in people at high-risk from Covid, and later in the broader population. By April 2022, more than 600 million doses of Pfizer/BioNTech’s mRNA-based Covid vaccine and nearly 150 million doses of Moderna’s vaccine had been administered in the European Union. But while this novel approach to vaccines came into its own during the pandemic, mRNA vaccine technology had initially been developed for use against cancer, with the aim of boosting the ability of the patient’s own immune system to recognise and attack tumour cells.
Before the pandemic, CureVac, founded in Tübigen, Germany, in 2000 ‒ the first of the ‘big three’ mRNA companies ‒ was testing an mRNA vaccine, combined with local radiation, in patients with non-small cell lung cancer. BioNTech ‒ a company based in Mainz, Germany, which started operations 2008 ‒ had been focused on developing therapies for melanoma, triple-negative breast cancer, head and neck cancer, lung cancer and KRAS-mutated solid tumours. Moderna ‒ the third big player, established in Cambridge, Massachusetts in 2010 ‒ was also investigating the use of mRNA vaccines in an oncological setting. “Back then, all of them tried to develop vaccines against cancer,” says Peter Brossart, Head of Haematology and Oncology at Bonn University Clinic, who was himself involved in some of the earliest clinical trials of mRNA vaccines in cancer patients, starting in 2003.
Then came Covid, and the rush to develop vaccines at speed and at scale that could offer reliable protection. All three companies turned their attention to developing mRNA vaccines against the SARS-CoV-2 virus. The performance of the Pfizer/BioNTech and Moderna vaccines showed what the new virus technology could do, in terms of efficacy, safety and speed of development. It also delivered huge commercial success ‒ the first for mRNA vaccines ‒ that could give a major boost to efforts to develop its use as a cancer therapy. “The companies have now the financial resources to develop and conduct vaccination studies again, to treat patients with malignant diseases,” says Brossart.
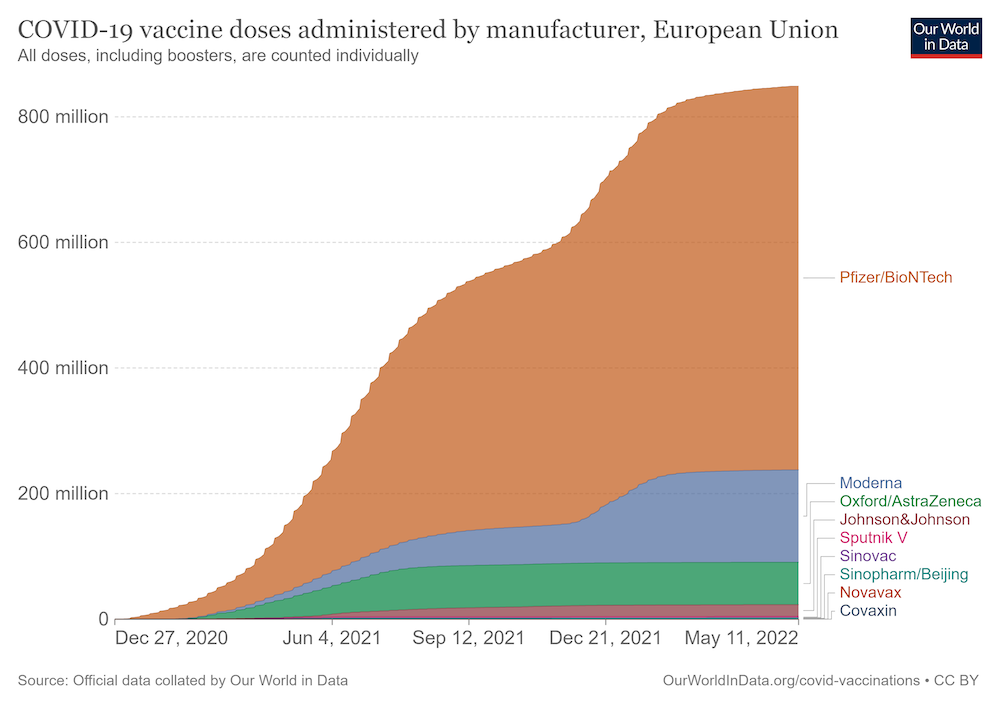
Source: Official data collated by Our World in Data, downloaded on 12 May 2022 from https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer?country=~European+Union Republished under a creative commons licence
mRNA cancer vaccine therapy: principles and history
The principle of using vaccines against cancer is to train the immune system to recognise tumour antigens and target them. The strategy has relevance in treating active disease as well as for adjuvant treatment, says Dirk Arnold, a gastrointestinal cancer specialist, and head of the Asklepios Tumour Centre in Hamburg, who is currently involved in a phase II trial of a BioNTech colon cancer vaccine. “At the moment, mRNA vaccines [in oncology] are clinically developed for two fields: to shrink existing lesions in highly immunogenic tumours and to prevent a relapse in minimal residual disease. In this adjuvant situation, the principle is similar to how Covid vaccines worked: a virus enters but the immune system is prepared and can kill it. In the oncologic situation, the disease recurs but now the vaccine strikes – it has trained the immune system for this situation and the tumour cells can be attacked.”
“The principle of using vaccines against cancer is to train the immune system to recognise tumour antigens and target them”
As Arnold points out, mRNA technology is a relative newcomer to the cancer vaccine scene. “Tumour vaccines have been studied a long time, especially with immunogenic tumours,” he says. The BCG vaccine, which is used primarily to prevent tuberculosis, also acts as immunotherapy for early-stage bladder cancer, where it has been approved and studied for decades. The first vaccine specifically developed as a therapeutic cancer vaccine, Provenge, was approved in 2010 to treat patients with prostate cancer. To train the immune system to detect cancerous cells with Provenge, dendritic cells and antigen presenting cells are collected from the patient. Outside of the patient, these cells are exposed to a protein intended to stimulate and direct them against prostate cancer cells. Finally, the immune cells are returned to the patient and should now be able to detect and fight cancer cells. “However, this can be made more effective with mRNA, which can show target structures to the immune systems more efficiently,” says Arnold.
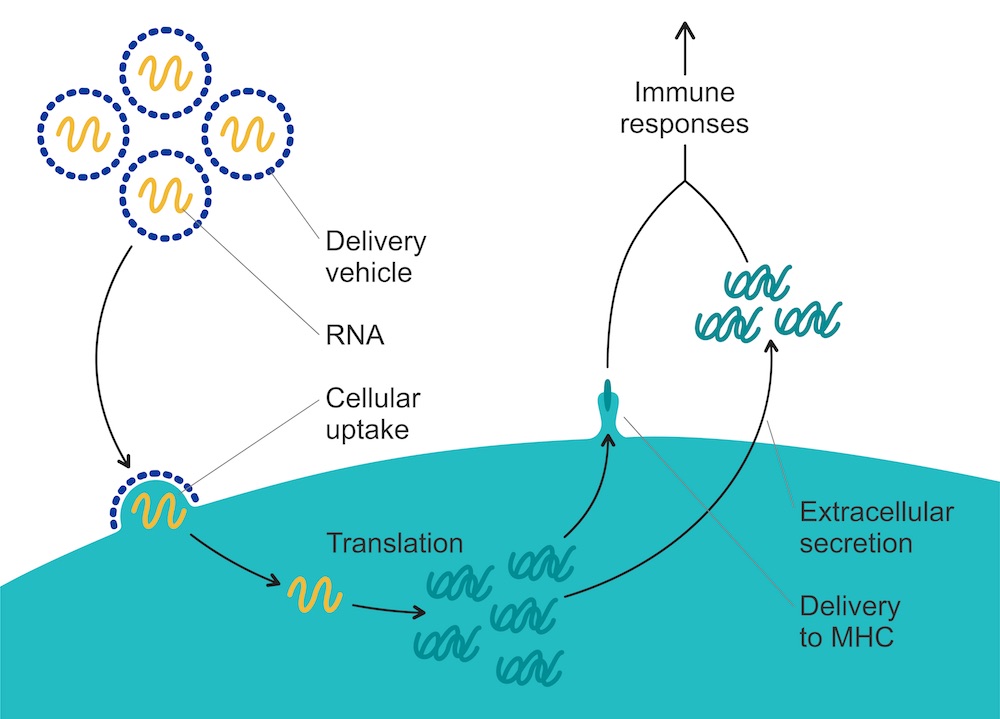
The concept behind mRNA vaccines is (deceptively) simple. During protein production in normal cells, mRNA is the messenger – hence its ‘m’. It ferries genetic messages that contain instructions for the building of various proteins which carry out all the functions necessary for life. To achieve this, mRNA is copied from DNA in the nucleus and delivered to ribosomes in the cytosol, where the protein is built.
mRNA vaccines can make use of this process by introducing mRNA that contains instructions to build proteins that code, for instance, for the production of the SARS-CoV-2 spike protein, or proteins expressed by cancer cells. When such ‘therapeutic’ mRNA is injected into the body, cells take up the nucleic acid. The cells are then coaxed into producing the desired protein or peptide from the introduced mRNA script. Among the cells likely to take up mRNA are dendritic and other antigen-presenting cells, which produce the encoded peptide and present it to immune cells, starting the adaptive immune response. mRNA vaccines can induce both an antibody-mediated response and T-cell responses. Unlike DNA, this mRNA doesn’t have to enter the nucleus to be transcribed, but instead goes directly to ribosomes for translation.
“There is a huge difference between inducing an effective immune response against a virus and doing the same against cancer cells”
Simple as it may sound, as Brossart emphasises, there is a huge difference between inducing an effective immune response against a virus and doing the same against cancer cells. Pathogens carry antigens that are foreign to the human body, and the immune system has evolved over millions of years to identify and eliminate them rapidly. Tumour cells, on the other hand, grow over a longer time, and have evolved mechanisms to evade the immune system. “It is more difficult to induce an immune response as the tumour cells’ milieu is often very immune-suppressive,” he says.
To induce an immune response at all, the vaccine needs to contain the right antigen to train the immune system. In earlier trials, vaccines used antigens that are selectively expressed or overexpressed in tumour cells but not in healthy cells. “Their advantage is that they are off-the-shelf vaccines,” says Brossart. “However, high-affinity T-cells recognising self-antigens will be eliminated more in the thymus.”
Most cancer vaccines currently tested instead follow a completely individual approach, where the vaccine is tailor made for each patient. A patient’s tumour is sequenced to find neoantigens ‒ a new protein that forms on cancer cells when certain mutations occur in tumour DNA, and is not expressed on healthy cells. The advantage of this approach, explains Brossart, is that neoantigen-specific T-cells will not be eliminated in the thymus. As the antigens are novel and foreign to the immune system, the response will also be stronger,” explains Brossart. “However, finding mutations and peptides to direct the immune response against is time-intensive.”
Arnold believes a middle ground is likely to be found between these two approaches. “One option is a vaccine targeting one feature, which can be given to a subgroup of patients. The other is a combined vaccine containing several target structures, with which a large intersection of patients can be treated.”
A rocky road
The ‘tangled history of mRNA vaccines’ was traced in an article in Nature in 2021. Brossart points to the seminal role played in the mid-1990s by Eli Gilboa and the team at the Center for Genetic and Cellular Therapies at Duke’s University, in Durham, North Carolina. They were among the first to investigate the potential for using mRNA that coded for proteins expressed by specific tumour cells to train the immune system to attack those same cells.
Their approach involved taking immune cells from the blood, then coaxing them, in vitro, to take up synthetic mRNA that encoded tumour proteins, after which they would be injected back into the body to marshal the immune system to attack cells expressing those proteins. Success in animal studies was followed by clinical trials using the same approach, but despite early signs of promise, they came to nothing.
The Eureka moment fell to a PhD student at the University of Tübigen, in Germany, by the name of Ingmar Hoerr. Having learned about the work of Gilboa, he experimented with injecting mRNA directly into mice intradermally – initially as a control. Surprisingly, the mRNA remained active in the cells for at least a little while ‒ enough to produce the antigen to raise an immune response against the protein. Unlike others, who had observed similar phenomena but then abandoned the line of research, Hoerr decided to pursue the mRNA approach further. In 2000, together with colleagues from his laboratory, Hoerr founded the company CureVac.
Based on these findings, Brossart, then a consultant in oncology and haematology at the University of Tübigen ran a trial using in vitro transcribed mRNA coding for several tumour-associated antigens to vaccinate patients with kidney cancer. That started in 2003 ‒ almost 20 years ago. “We injected RNA intradermally into patients. It worked partly and we observed clinical relevant remissions in some patients – but partly not,” Brossart recalls. He and his group collaborated with CureVac, who produced the mRNA without any of the modifications and stabilising procedures that have since been developed. “Already then we could see an immune response from patients against the vaccination. In several patients, symptoms improved and the tumours shrank in size,” says Brossart.
“We received no more funding for our study, because no one believed that immune therapy could work against cancer”
It was a good start, but there were clearly many technical challenges ahead, on top of which, the whole enterprise suffered from a severe lack of commercial confidence around immunology approaches to treating cancer, which had taken a knock following a series of disappointments. “There came a time during which people stopped believing in immune therapy,” Brossart recalls. “We received no more funding for our study, because no one believed that immune therapy could work against cancer.”
It took the success of checkpoint inhibitors to rebuild that confidence. The immune system was once again seen as an ally in combatting cancer, says Brossart. “Checkpoint inhibitors revitalised and revolutionised this field.” He adds, though that the success of checkpoint blockade somewhat overshadowed the potential value of cancer vaccines. “People asked: what role would mRNA-based vaccines play? But not all patients respond to checkpoint inhibitors, many relapse. There are many reasons for this, and cancer vaccines could provide an additional tool to stimulate an anti-cancer mediated immunity in these patients.”
Before that could happen, however, there were a number of technical problems to be solved. Key among them lay in the nature of RNA itself. RNA is unstable and rapidly degraded by the ubiquitous RNase. Not only that, as soon as mRNA is injected into the body, it is easily destroyed. During evolution, immune systems have learned that foreign mRNA only belongs to viruses and other pathogens, so our bodies immediately attack mRNA molecules.
A lot of work was done to develop clever ways to smuggle mRNA into cells, and coaxing cells into producing significant amounts of peptide
In the years between the very partial responses that Brossart and colleagues were able to show in kidney cancer patients, using unstabilised, unmodified mRNA, and the success shown by the mRNA vaccines during the Covid pandemic, a lot of work was done to develop clever ways to smuggle mRNA into cells, and coaxing cells into producing significant amounts of peptide.
Optimizing mRNA
First of all, mRNA needs to be brought into cells – which isn’t that easy. Naked mRNA is unstable and quickly degraded, so mRNA is now protected on its way to cells by formulating it into ‘delivery vehicles’, including lipid nanoparticles and polymers, that protect the mRNA until it reaches its site of action. Lipid nanoparticles were used to deliver both the BioNTech/Pfizer and the Moderna vaccines against Covid.
mRNA is also immunogenic, as it is recognised by a variety of pattern recognition receptors, which have evolved to detect single- and double-stranded RNA molecules from microbes and block mRNA translation. Pattern recognition receptors also activate the interferon-related pathway and elicit innate immunity, which inhibits antigen expression and dampens the immune response.
To reduce this inflammatory response, researchers have tinkered with the mRNA itself. Biochemist Katalin Karikó and immunologist Drew Weissmann, both at the University of Pennsylvania in Philadelphia, found that mRNA could be altered to reduce its immunogenicity, by replacing the nucleotide uridine with an alternative nucleotide, pseudouridine. Pseudouridine is similar to uridine, but contains a modification. Using pseudouridine not only decreases the anti-RNA immune response, but also enhances RNA stability. This technology is licensed by both BioNTech and Moderna and found its way into their Covid vaccines. CureVac, on the other hand, does not replace uridine with pseudouridine. Instead, it alters the mRNA sequence so that the protein it codes for doesn’t change, but minimises the amount of uridine used.
Lipid nanoparticles, pseudouridine and other modifications all contributed to the success that was seen in the mRNA-based Covid vaccines, which is generating huge interest in what the technology may be able to achieve with the various cancer vaccines that are currently in trials.
mRNA cancer vaccines: current strategies
Much remains to be clarified about where cancer vaccines can find their most effective place within therapeutic strategies, and the right choice of target. In the phase II trial of BioNTech’s vaccine BNT-122, the vaccine is administered to patients who have received surgery and chemotherapy for colon cancer, but test positive for circulating tumour DNA (cell-free tumour DNA), which points to minimal residual disease, and a high risk of relapse.
“If patients present with cell-free tumour DNA despite surgery and chemotherapy, there is likely an occult tumour, which will be targeted by the vaccine,” says Arnold, the GI cancer specialist who heads up one of the many participating cancer centres, in Hamburg. Currently, he says, the first patients are still receiving chemotherapy, after which the mRNA vaccine will be given to those showing signs of minimal residual disease.
The BioNtech vaccine will be tailor made based on the specific neoantigen expressed by each patient’s individual tumour.
Findings from that individualised approach can inform such future approaches, says Arnold. “We will not only see which target structures are there, but also whether they are suitable for targeting by vaccines.” For example, relapses might occur less frequently in patients in which one antigen was targeted, while other antigens might mutate and evade targeting. “We will need to find stable, robust features, which don’t change rapidly.”
An alternative approach is being taken with the vaccine for HER-2 positive breast cancers pioneered by Herbert Lyerly, at Duke University. According to reporting by Science Focus, the vaccine will be administered to patients while they are receiving treatment with Herceptin, and rather than being tailor made for each patient, it will target four known mutations that arise in patients with HER2-positive breast cancer, in which the tumours have evolved mutations resistant to Herceptin. In a study to be started in 2022, patients with advanced HER2-positive breast cancer who are not yet resistant to Herceptin will receive the same vaccine targeting these four mutations, which they are expected to develop in the course of treatment. “We’ll effectively be vaccinating people against mutations that their cancer doesn’t yet have,” Lyerly tells Science Focus.
When cancer cells harbouring these mutations do appear, the immune system is expected to recognise and destroy the mutant cells. In this case, the tumours will remain sensitive to Herceptin and patients can continue to be treated with the drug.
That pre-emptive approach may foreshadow a time when cancer vaccines are even developed for a preventive setting, for people deemed to be at high risk. Though that may still be a long way off, Arnold argues that prophylaxis for heritable tumours is something “very conceivable” and is eagerly anticipated. “If we have clear, unique target structures, it is imaginable that we can reduce risk of disease.”
With less immunogenic tumours like colon cancer, the goal is to keep recurrence or minimal residual disease in check
How much can realistically be expected of mRNA vaccines, he adds, will depend on the tumour. For highly immunogenic tumours, like renal cell cancer and melanoma, Arnold expects that existing tumours might be shrunk or controlled using a vaccine. “With less immunogenic tumours like colon cancer, the goal is to keep recurrence or minimal residual disease in check,” he says.
And as Brossart adds, though currently trialled as monotherapies, cancer vaccines may well also be combined with other therapies. “Combinations with checkpoint inhibitors are one possibility, which could enhance the immune system’s function. Some data indicate that this might be the case.”
Looking ahead
These are exciting time in the cancer vaccine space, but Brossart warns that side effects, long term effects and efficacy of mRNA-based cancer vaccines will have to be established, particularly before testing in a prophylactic setting. That said, as a result of their use in the pandemic, we now have copious and robust data on the side effects of mRNA vaccines, and ‒ especially in the context of cancer ‒ the data look good, says Arnold. “From the many millions of mRNA vaccinations to prevent Covid, we know the side effect profile: flu-like symptoms with very rare endocrine side effects and myocarditis. These will also occur when treating tumours, but measured against the advantage, these side effects are in line with the benefits to be expected.”
Despite mRNA’s success in the Covid pandemic, it might still take some time until mRNA becomes a standard therapeutic molecule in oncology. “Studies in which a tumour is present and its size is reduced through vaccination – such as in melanoma or renal cell – this might be rapid, as one should be able to quickly judge success,” says Arnold. “In the adjuvant setting, we have to show that relapses don’t occur or occur more rarely. Just waiting for this endpoint means that the studies will take longer.” Such trials recruiting now will have definitive results in three to four years, Arnold estimates – eagerly awaited results.