Adverse effects of cancer therapies have long been at the root of decision making for interventions in all modalities – surgery, radiotherapy and medical – and especially in the latter category of anti-cancer drugs. Most people have heard of some side-effects, often severe, of cytotoxic chemotherapies, but the advent of targeted and biological therapies in the past 20 years promised not only greater precision in treating cancers but also fewer and less-severe side-effects. That promise has not been met with many new agents.
In the cancer drug landscape, the first departure from traditional chemotherapies is widely seen to be the introduction of the class of small-molecule inhibitors such as imatinib (Glivec – a drug that had a huge impact on oncology). Some of these inhibitors, which are essentially chemicals, can also have side-effects as bad as chemotherapies, albeit with different types of effects according to the agent (the common toxicity criteria for adverse events of cancer drugs is 1=mild, 2=moderate, 3=severe, 4=life-threatening, 5=death).
The rest of the new drugs and approaches now in play are biological therapies, with the main ones widely deployed over the past 20 years being three types of monoclonal antibody. One group comprises targeting immunotherapies such as cetuximab (targeting EGFR) and trastuzumab (targeting HER2). Another group comprises anti-angiogenesis drugs such as bevacizumab (anti-VEGF). The targeting immunotherapies can have severe adverse effects on the cardiovascular system and such events have been comprehensively reported and helped give rise to the field of cardio-oncology.
But a more recent class of monoclonal antibodies that regulate the immune system – checkpoint inhibitors such as ipilimumab – have raised particular concerns about a diverse range of adverse events.
There are also other new and experimental immunotherapies such as CAR T-cell therapies, vaccines and oncolytic viruses. (In fact the first immunotherapy found to be effective is the cytokine IL-2, but at high doses adverse events can be so severe that it needs to be given in an in-patient setting.)
The flood of new agents, and especially the rapidly growing interest in the immune system, has led to a big expansion in possible side-effects of treatment, resulting from novel mechanisms of action and dose-response characteristics that differ from standard therapies. This has placed major demands on oncologists and the teams around them. Great pressure has also been placed on regulators and health technology assessors in assessing and following up the efficacy‒safety balance. A stream of papers is constantly adding to the literature about adverse events, and initiatives to capture patient reported outcomes and experiences, as well as real world evidence, are playing catch-up with new treatments as they become widely deployed.
Checkpoint inhibitors in the spotlight
Checkpoint inhibitors have revolutionised cancer therapy, as they have achieved previously unattainable success in slowing progression and extending survival in some patients, in particular for melanoma, lung cancer and certain types of colorectal cancer, with many applications and combinations under study. They are of concern, however, because of the many unpredictable immune-related adverse events that can be life-threatening.
The first agent, ipilimumab, a CTLA-4 inhibitor, was approved by the US regulator, the FDA, in 2011. CTLA-4 is a protein on T cells ‒ a type of immune cell ‒ that stops the cells from eliminating cancer and other cells. Inhibiting (blocking) CTLA-4 therefore increases the T cell’s ability to kill cancer cells. Since ipilimumab was approved, only one other CTLA-4 drug has made some progress (an FDA orphan designation for use in a combination).
There are six checkpoint inhibitors on the market ‒ including pembrolizumab and atezolizumab ‒ that target the PD-1 protein (located on T cells) or PD-L1 protein (located on cancer and other cells). Binding between the two proteins stops T cell activation, so the drugs target one or the other to release the T cells to do their work.
The 2018 Nobel Prize in Physiology or Medicine was awarded for the discovery of CTLA-4 and PD-1 checkpoint inhibition, cementing interest in the new agents.
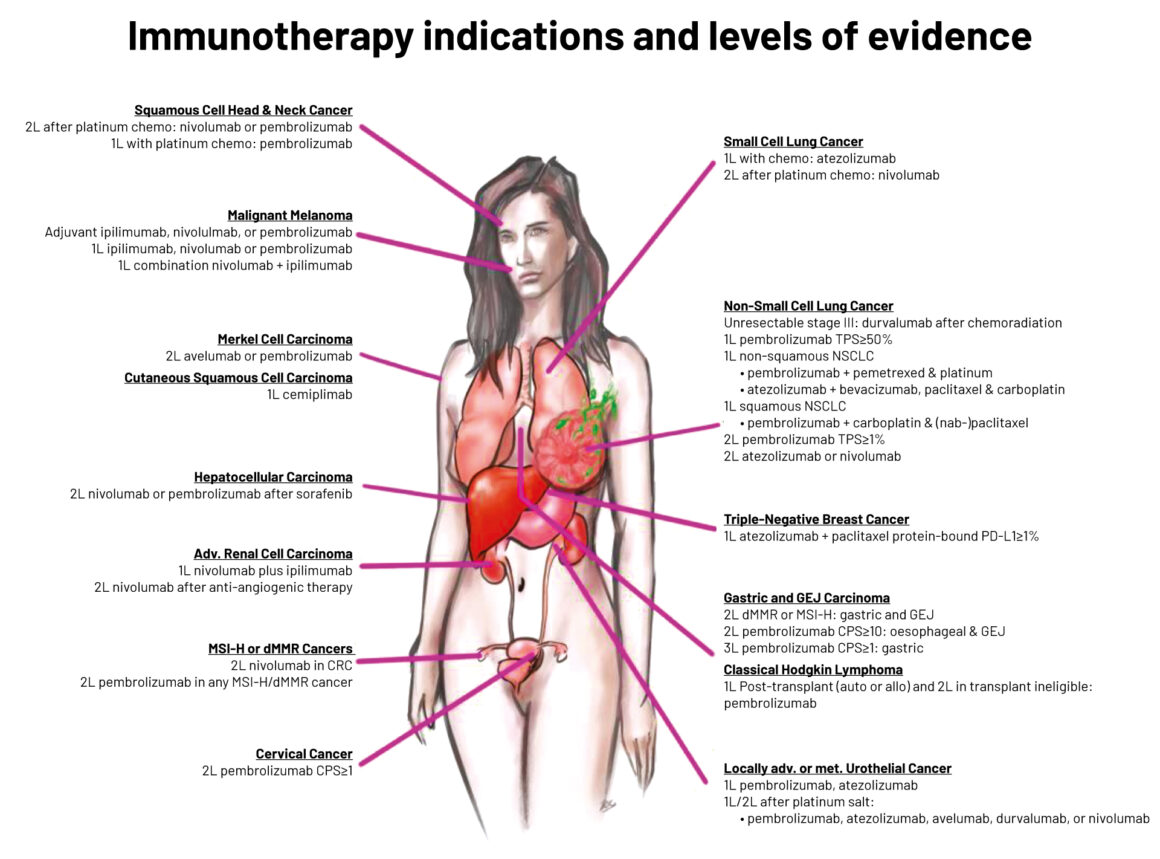
Legend: L1 indicates uniform NCCN consensus that the intervention is appropriate based on a high level of evidence; L2 indicates uniform NCCN consensus that the intervention is appropriate based on a lower level of evidence; L3 indicates there is major NCCN disagreement that the intervention is appropriate, based on any level of evidence
MSI-H ‒ microsatellite instability-high; dMMR ‒ deficient mismatch repair; CRC ‒ colorectal cancer; TPS ‒ tumour proportion score; NSCLC non-small-cell lung cancer; GEJ ‒ gastro-oesophageal junction; CPS ‒ combined positive score; PMBCL ‒ primary mediastinal large B cell lymphoma; R/R ‒ relapsed/refractory
Source: Raju K Vaddepally et al (2020) Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 12:738. Republished with kind permission of Raju K. Vaddepally © 2020 Raju K. Vaddepally
What happens when you take the brakes off?
The often spectacular success of these agents in a cohort of patients has led to their speedy introduction in the clinic, but there are clearly major gaps in knowledge.
Reviews of side-effects of cancer drugs have generally found that immunotherapies are safer and better-tolerated than standard or targeted therapies, but criteria for measuring toxicities in studies may not have covered all the immune-related adverse effects (known as irAEs) and some may have been underestimated. That is confirmed in a flurry of recent papers on checkpoint inhibitors that have described the many irAEs that can affect nearly all of the body’s organs, their management, and to some extent the mechanisms thought to be responsible for this wide spectrum of unwanted effects.
In a review looking back at 10 years of checkpoint inhibitors, Caroline Robert, head of dermatology at Gustave Roussy, the cancer centre in Paris, writes that, by inhibiting the ‘brake’ of immune activation, these drugs “often have off-target effects resulting in immune-mediated inflammation of diverse organs or tissues.” Put simply, these drugs are not targeting cancer cells directly, and the stimulation of the immune system can create inflammatory attacks on healthy tissue in many organs.
It’s a pressing issue also because of scale. Robert notes that these drugs have already become some of the most widely prescribed anticancer therapies, and there are more than 3,000 active clinical trials evaluating T cell modulators, which is about two-thirds of all oncology trials.
“Their pathophysiology is poorly understood, with several proposed mechanisms leading to organ damage”
The many new reviews on irAEs are aiming to fill gaps in knowledge. As the authors of one on the multi-organ irAEs of atezolizumab, an anti-PD-L1 inhibitor, note: “Although guidelines exist for the diagnosis and treatment of ICI [immune checkpoint inhibitor] related toxicities, there is no international consensus on the terminology used for their definition, diagnostic criteria, grading and causal attribution, and little evidence supporting their management… Their pathophysiology is poorly understood, with several proposed mechanisms leading to organ damage.”
That irAEs have also attracted the attention of emergency physicians in a number of papers indicates that this new and unpredictable class of drug will increasingly need awareness of symptoms and management strategies among health professionals beyond, as well as within, the oncology community.
Melanoma advocates flag concerns
Melanoma patients have been in the frontline for new agents, not just immunotherapy but also targeted inhibitors, which have transformed the treatment of advanced disease. But as Gilly Spurrier-Bernard, vice-president of Melanoma Patients Network Europe (MPNE), says, patients often have to navigate the evidence about adverse events themselves, particularly those treated outside of comprehensive cancer centres ‒ though not even cancer centres necessarily have access to all the expertise needed. “As immunotherapies have a number of side-effects, some rare, that affect a range of organs, patients may need to be seen by specialists such as rheumatologists, endocrinologists, cardiologists and others, and they need confidence to voice these needs,” she says.
In large countries such as France, where Spurrier-Bernard is based, local hospitals may have only basic multidisciplinary oncology teams; an expert centre with access to specialists may be a long distance away. In advocacy work with MPNE and also MelanomeFrance, she and colleagues have been busy consulting guidelines, trial papers and databases of adverse events, such as VigiAccess, to help patients ask the right questions. As she points out, these drugs are mostly ‘black triangle’ labelled, meaning they are subject to additional monitoring by the European Medicines Agency.
“Some patients are being told that side-effects are not to do with their treatment, but guidelines show they can be”
“Some patients are being told that side-effects are not to do with their treatment, but guidelines show they can be,” says Spurrier-Bernard. “And a patient I spoke to recently was told that side-effects would be the same in switching to targeted from immuno after progression ‒ and that was an oncologist. We informed them that there may be differences.”
She notes a number of other issues that concern immunotherapies, such as patients mistakenly being treated for pneumonia with antibiotics ‒ which can also lessen treatment efficacy ‒ instead of for immune-induced pneumonitis. The sometimes late appearance of side-effects can also be particularly challenging, now that immunotherapy is being given in the adjuvant setting. Rare side effects can be difficult to diagnose. Added complexity arises when patients suffer toxic effects on more than one organ system at the same time or serially, and from the increasing use of combinations, both with other immunotherapies and also with other drug types. Long-term management of irAEs may also be needed.
Additional comment from the melanoma perspective was provided by MPNE founder Bettina Ryll at an event last Autumn, ‘Cancer therapy: Pre-empting the future epidemic of immune-related adverse events’, hosted by the British Society for Immunology (BSI) and the National Cancer Research Institute (NCRI). She noted other symptoms that have been missed or misdiagnosed, such as colitis and endocrinological dysfunctions, and that immune-related fatigue is being “severely underestimated”. She also mentioned the Netherlands Pharmacovigilance Centre (Lareb), which is another knowledge centre for adverse drug reactions. Patients, she said, cannot assume their healthcare providers are aware of irAEs.
Toxicity affects a wide range of organs
The side-effect profile of checkpoint immunotherapies has been well described in the literature. The most common effects are skin and gastrointestinal toxicities; also common are those that affect the endocrine, pulmonary, hepatic and musculo-skeletal systems. Less common ‒ or uncommon ‒ are those that are neurological, cardiac, renal, ocular and haematological in nature. It’s also known from trials and case reports that some irAEs are life threatening or fatal, and now there are reviews of emergency presentations that are giving a better picture of how these drugs are likely to impact the healthcare community, as the number of patients and agents rise.
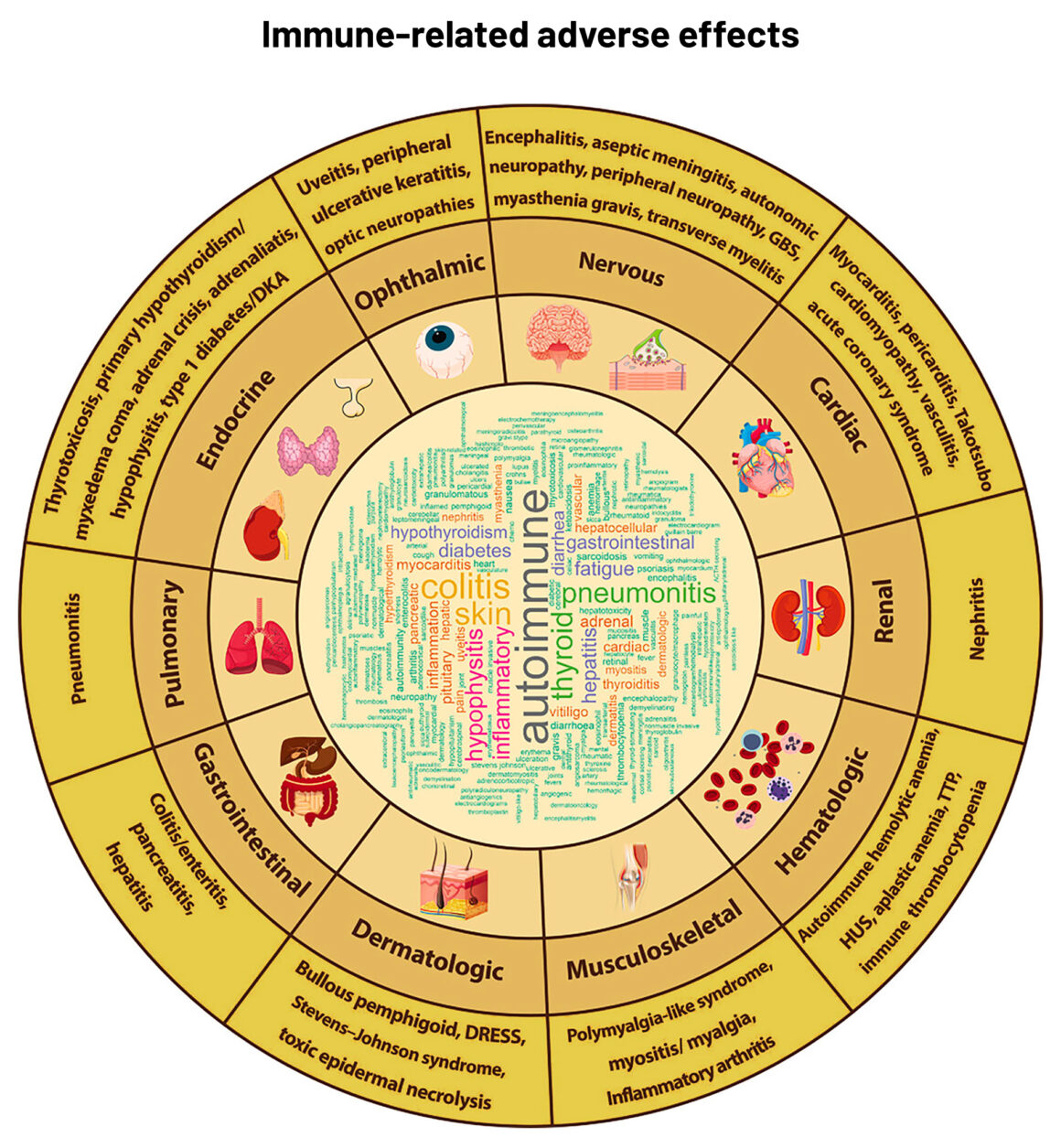
Abbreviations: DKA ‒ diabetic ketoacidosis; DRESS ‒ Drug Rash with Eosinophilia and Systemic Symptoms; GBS ‒ Guillain-Barré syndrome; HU ‒ haemolytic uremic syndrome; TTP ‒ thrombotic thrombocytopenic purpura
Source: S-CJ Yeung (2020) Diagnosis and management of immune‐related adverse effects of immune checkpoint therapy in the emergency department. JACEP Open 1:1637–59. Republished under a Creative Commons Attribution‐NonCommercial‐NoDerivs License
Take the Christie cancer centre in Manchester, UK, which has an assessment unit that receives emergencies. In a study of 300 patients being treated with checkpoint inhibitors seen at the unit, 98 were diagnosed with an immune-related toxicity, most commonly colitis, hepatitis and pneumonitis; these patients had an average in-patient stay of 7.1 days, and two died within 7 days. Most of the 300 were being treated for melanoma, and lung and renal cancers.
In a similar study, this time at the emergency department of the MD Anderson Cancer Center in the US, more than 1,000 emergency visits were identified for 628 patients on checkpoint therapy, and about a quarter of the visits related to one or more irAEs. The authors found the most common irAEs were diarrhoea, colitis, pneumonitis, hypophysitis (pituitary inflammation), and dermatitis, but they also found variability according to the inhibitor or inhibitor combination, with significant variability in diarrhoea, hypophysitis, thyroiditis, and pancreatitis. This study also followed up on survival, noting poor outcomes for pneumonitis, but patients with colitis fared better.
Both of these studies took place in world-leading cancer centres but, even so, identifying irAEs may be challenging. The Christie authors note that, although irAEs are common and must be suspected, a majority of patients do not have them on presentation, and correct diagnosis is vital to start treatment such as steroids ‒ the mainstay first-line therapy. They note that while some patients may need steroids for other conditions, there is a danger of overtreatment if irAEs are not correctly identified, and also the timing and dosing of steroids for life-threatening toxicities is not clear at present. They confirm that antibiotics may also be given that could reduce the effectiveness of inhibitors.
It points to the need for resources to do a thorough clinical work up, which is an issue for emergency departments and for surveillance
What this points to is the need for the resources to do a thorough clinical work up, which is an issue not just for emergency departments but in ongoing surveillance of patients treated with checkpoint inhibitors. It also concerns a range of organ specialists as well as oncologists, who are all being briefed in reviews on checkpoint inhibitors in their specialist journals.
Bringing in organ specialists
The input of organ specialists with an immunology interest will be critical in understanding why irAEs occur and how they can be managed, and better drugs developed.
A paper that discusses how the field can move beyond generic and sometimes harmful treatments for immune-related adverse events is by Khashayar Esfahani and colleagues, Moving towards personalized treatments of immune-related adverse events, in Nature Reviews Clinical Oncology. They say that, too often at present, urgent clinical needs dictate a ‘best educated guess’ for management by oncologists, owing to limited evidence in current treatment guidelines and articles. While steroids are a cornerstone of first-line treatment, they propose a personalised approach for severe events where the immunohistopathology of affected organs is obtained, at a minimum, through biopsy, which can guide targeted therapies. Where possible more detailed analysis of mechanisms should be obtained, such as assessment of blood cytokines. Much of what they propose lies in the research agenda, and requires collaboration among oncologists, organ-specific autoimmunity specialists and basic scientists. What seems to be certain is that successful interventions, especially in severe and uncommon irAEs, will require ‘ultra-specialised’ multidisciplinary care.
Speaking at the BSI/NCRI event, Lucia Possamai, a hepatologist at Imperial College, London, homed in on hepatitis, describing distinct and complex liver conditions caused by checkpoint inhibitors, noting that combination therapies have a much higher adverse event rate than single therapies in melanoma and also the most severe grade irAEs for any organ. Hepatitis accounts for a major proportion of irAE fatalities, in the range of 7‒20%.
Possamai pointed out that current treatment strategies are not evidence based and do not target the liver, let alone the mechanism causing hepatitis, relying instead on steroids, which themselves have side-effects, and may affect the efficacy of immunotherapy. Second-line MMF (mycophenolate mofetil) immunosuppressants ‒ which act to inhibit T and B cell proliferation ‒ have slow onset and may also affect the action of checkpoint inhibitors.
There’s a packed agenda for resolving these issues that requires research among oncologists, immunologists and organ specialists
There’s a packed agenda for resolving these issues, said Possamai, that requires research among oncologists, immunologists and organ specialists. It includes recognising that a variety of mechanisms may be in play in organ toxicity; finding the ‘holy grail’ that can uncouple the effectiveness of inhibitors from irAEs (which may be done through better drugs and personalised irAE treatment strategies); and predicting and preventing toxicities. In the meantime there are ways to minimise the side-effects of steroids, including using drugs optimised for certain organs. Possamai noted progress made in treating irAE colitis with the drugs vedolizumab and infliximab that target, respectively, the organ and pathway.
Certainly a critical issue is identifying the small number of patients who will benefit from immunotherapy. Finding biomarkers that predict response, however, is said to be like ‘finding the needle in haystack’.
Other organ specialists are also weighing in with briefings. A key point is that, even if certain irAEs are not common, they can still be life threatening, such as pneumonitis, and respiratory physicians have stepped into publish management guidance of pulmonary toxicities, which have been identified in trials.
Severe cardiac irAEs are rare and were not seen in the key trials that led to approvals of checkpoint inhibitors, probably owing to small patient numbers. Even the authors of the MD Anderson study, noted above, only saw one case of non-fatal myocarditis in their large sample. But cardiologists are rightly concerned about evidence of cardiotoxicity that has emerged in case reports and some later trials and there is already considerable literature on this aspect of irAEs, such as from a team in Germany and in the UK, and in this latest systematic review. Cancer World reported a study recently that found cardiotoxicity is higher than previous estimates.
Although the field of cardio-oncology has a 15 year history, the latest round of adverse events from new drugs may have spurred new interest from the cardiology side. The European Society of Cardiology set up a cardio-oncology council in 2018, and it has been called a new subspeciality by a group in the UK. Some centres are only now setting up cardio-oncology units.
Dermatologists are reporting a surprising finding with irAEs – that skin toxicity can actually be an indication that the drugs are effective (see for example Alessandro Allegra et al 2021); and other irAEs may also be associated with better prognosis.
Hypophysitis is described as “an intriguing adverse event with many faces” with an “elusive biological background”, and requiring increased awareness and constant vigilance in a multidisciplinary approach to ensure optimal care. Rheumatologists are being alerted that awareness of side-effects can be particularly important in patients with pre-existing autoimmune conditions.
Another concern is the impact of immunotherapies on older people, given that trials often focus on younger, fitter patients, as a position paper on non-small cell lung cancer from the International Society of Geriatric Oncology discusses.
Towards models of care
There are then dozens of reviews of irAEs and also guidelines from ESMO, ASCO and the US National Comprehensive Cancer Network, plus a guide for patients by ESMO (for which Gilly Spurrier-Bernard is a co-author). There is even a free mobile phone app, IO TOX, for Apple and Android phones, based on the ASCO guideline. The crucial question is how all this advice is translating into practice.
On the frontline, the rush to research and implement immunotherapies has resulted in many new immuno-oncology centres in institutes and cancer centres, but much of this effort does not include irAEs and the multidisciplinary teams needed to manage everyday clinical practice. There are initiatives that are helping and could serve as models. The Dutch Melanoma Treatment Registry, for example, is more than just a database; since 2013 it has aimed to audit how new drugs work and has recorded every patient treated with advanced melanoma in the Netherlands, as all expert centres participate. Studies report ‘real world’ outcomes including irAEs of new therapies (see for instance Van Zeijl et al. 2021).
In the UK, advanced melanoma should only be treated at a specialist skin cancer unit. Minimum case volumes have also been specified in some countries for treating patients with advanced disease, such as 40 a year for skin cancer units in Germany, with a view to encouraging the consolidation of expertise in high volume centres.
Gustave-Roussy in Paris, one of France’s top cancer centres, has set up ImmunoTOX (iTox) ‒ a multidisciplinary board that has been meeting twice a month and comprises organ specialists, oncologists, drug specialists and radiologists to help prescribing physicians manage difficult or unusual irAEs. In a report covering a period from 2016 to 2019, the board looked at 356 patients, of whom 273 were found to have irAEs, a majority at grade 3‒4.
The RETOINMUNO group brings in a full array of organ specialists plus intensive care specialists and also nurses
A group at Barcelona’s Hospital Clinic set up a multidisciplinary team in 2018 to coordinate management of patients with suspected irAEs. The group, called RETOINMUNO, now gives patients individualised care, bringing in what seems to be a full array of organ specialists, plus intensive care specialists and also nurses, who are said to be vital in detecting irAEs and educating patients on treatments. A recent paper by the group details mainly the current state of play of treating irAEs, but also provides some background on multidisciplinary aspects including a workflow.
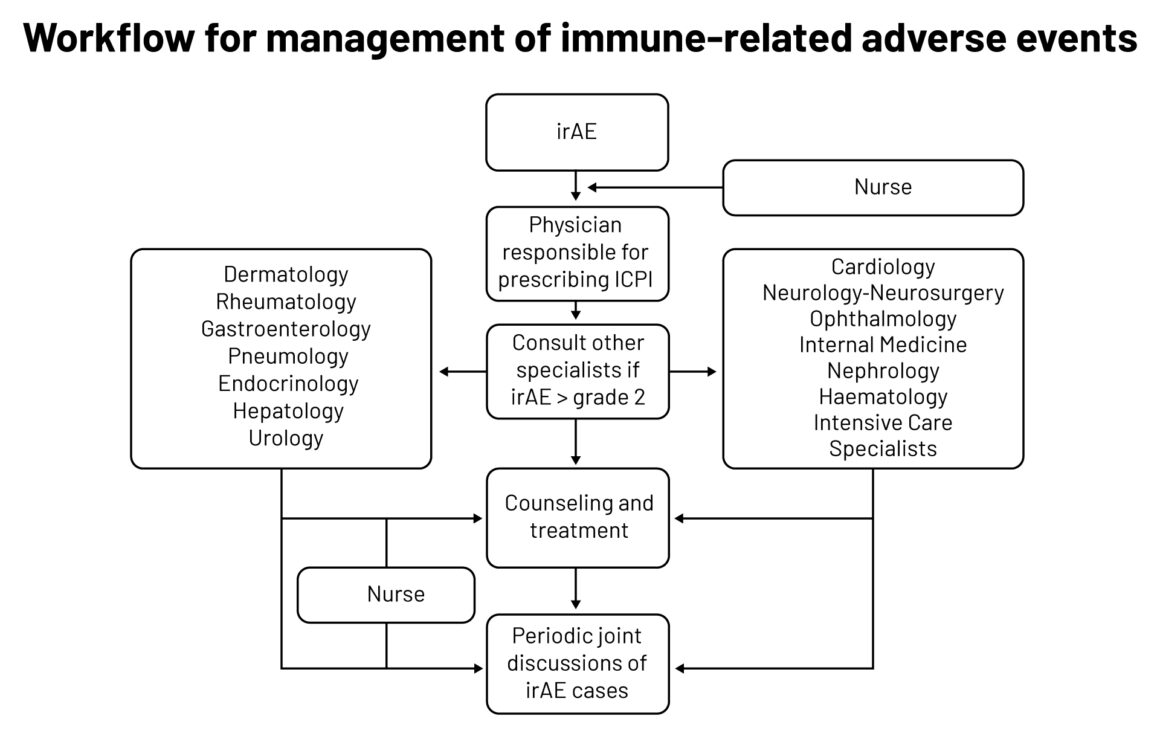
Legend: irAE ‒ immune-related adverse events; ICPI ‒ immune checkpoint inhibitors
Source: MC Londoño and M Reig on behalf of the RETOINMUNO Multidisciplinary Group (2020) Multidisciplinary Clinical Approach to Cancer Patients with Immune-Related Adverse Events Induced by Checkpoint Inhibitors. Cancers 12:3446
Republished under a Creative Commons Attribution License
Awareness is a big part of the picture, as a Lancet Oncology paper from 2020 described, covering physician and patient education that could maximise the chances of appropriate toxicity management. Oncologists and their extended teams, including advanced practitioners (who may be nurses) and pharmacists, are mentioned as arguably the most important group to educate as immunotherapy gains new indications in more cancer types.
Establishing such centres of excellence in immunotherapies around Europe could help extend education and expertise through networks that are accessible to patients and physicians throughout a country – and so improve the balance between effectiveness and safety that checkpoint inhibitors will increasingly demand.
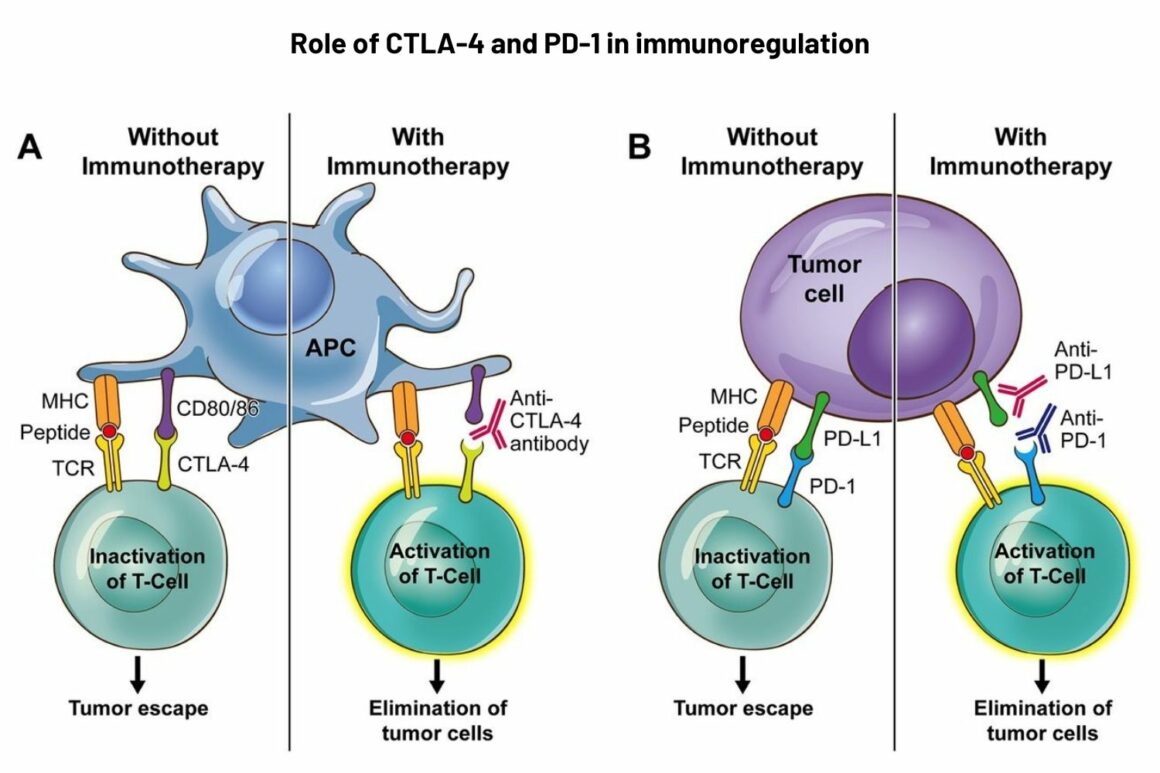
Panel B: PD-1 is expressed on exhausted T cells, B lymphocytes, natural killer cells, such as tumour-infiltrating lymphocytes, including monocytes and dendritic cells. PD-1 pathway inhibits signalling downstream of the T-cell receptor (TCR). PD-1 has two ligands: PD-L1 and PD-L2. PD-1 blockade by monoclonal antibodies (nivolumab and pembrolizumab) can provoke a peripheral, antitumour immune reaction.
Legend: MHC ‒ major histocompatibility complex
Source: E Soularue et al. (2018) Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 67:2056‒67. Republished with permission. © 2018, BMJ Publishing Group Ltd & British Society of Gastroenterology
Cover illustration by: Maddalena Carrai