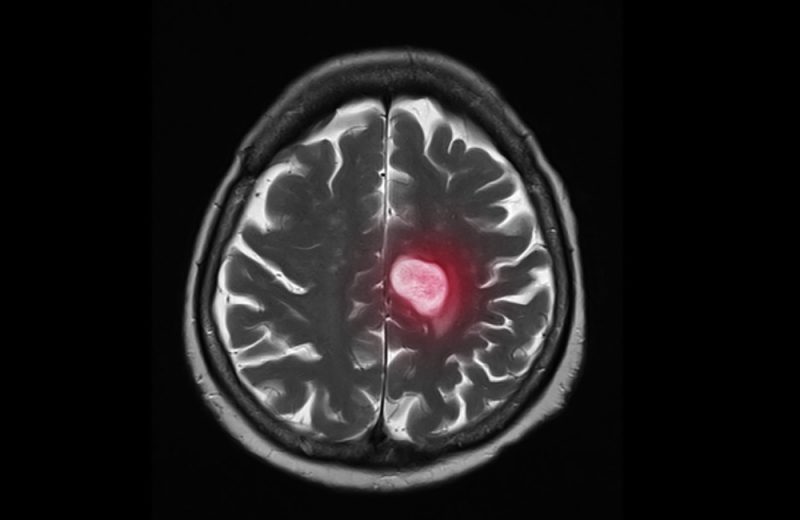
The antibody drug conjugate trastuzumab deruxtecan (T-DXd) showed substantial intracranial activity in patients with HER2-positive breast cancer whose disease had metastasised to the brain. The DESTINY-Breast12 study, presented at the Congress of the European Society for Medical Oncology (ESMO), held 13–17 September in Barcelona, (Abstract LBA18), and published simultaneously online in Nature Medicine, showed that in patients who had brain metastases at the start of the study, the 12-month overall progression free survival rate was 61.6% and the median progression free survival was 17.3 months.
“Results from DESTINY-Breast12 support the use of T-DXd for patients with HER2+ breast cancer irrespective of the presence of stable or active brain metastases,” said Nancy Lin, the study presenter from the Dana-Farber Cancer Institute, Boston. DESTINY-Breast12, she added, represents the largest prospective study yet reporting intracranial activity for T-DXd in patients with HER2+ metastatic breast cancer and brain metastases.
Around half of patients with HER2-positive metastatic cancer go on to develop brain metastases, which are associated with higher morbidity and shorter survival. Neurosurgery, radiosurgery, and whole-brain radiotherapy are often used to treat brain metastases; however, these techniques can lead to neurologic toxic effects that reduce the patient’s quality of life, and the disease usually progresses within six to 12 months of treatment.
Although regimens based around the kinase inhibitor tucatinib have shown efficacy, the median progression free survival found in the HER2CLIMB study (adding tucatinib to trastuzumab and capecitabine), published in the New England Journal of Medicine in 2019, was 7.6 months. Such data clearly demonstrates the current unmet medical needs of patients with HER2 positive brain metastases.
T-DXd consists of the chemotherapy agent deruxtecan linked to the antibody trastuzumab, which targets the HER2 protein on breast cancer cells. On the basis of the phase 3 DESTINY-Breast03 study, which showed a progression free survival at 12 months of 78.8% for patients taking T-DXd versus 34.1% for trastuzumab emtansine, T-DXd was approved for use in metastatic HER2-positive breast cancer. However, one of the most important unknowns with respect to T-DXd was its performance relative to the tucatinib-capecitabine-trastuzumab combination in patients with brain metastases. Patients with brain metastases had only been eligible for DESTINY-Breast03 if their metastases had been previously treated and were stable; those with symptomatic brain metastases were excluded. Several small prospective clinical trials and case series have supported high rates of central nervous system responses with T-DXd, but the total number of patients treated was small. “There is a need for additional prospective data, particularly in patients with active brain metastases, with respect to T-DXd,” said Lin.
For the phase 3b/4 DESTINY-Breast12 study, patients with HER2-positive metastatic breast cancer who had progressed on up to two lines of therapy were divided into two cohorts. The first cohort (n=263) consisted of patients with baseline brain metastases, including those with previously treated stable brain metastases (n=157) and active (untreated or previously treated and progressing) brain metastases (n=107). The second cohort (n=241) consisted of patients with no evidence of brain metastases at baseline. In both groups T-DXd was administered intravenously every three weeks (21-day cycle) at a dose of 5.4mg/kg of body weight until disease progression (measured according to RECIST 1.1) occurred outside of the central nervous system. Patients with baseline brain metastases received no more than 3mg dexamethasone daily, or its equivalent, for symptom control. Patients were treated at 78 cancer centres across Western Europe, Japan, Australia and the US.
In the cohort of patients with baseline metastases, the primary endpoint was progression free survival (PFS). The 12-month PFS rate was 61.6%, the 12-month central nervous system PFS rate was 58.9%, and the median PFS was 17.3 months. For patients with stable and active metastases, results were consistent. Patients with stable brain metastases had a 12-month PFS rate of 62.9% and a 12-month central nervous system PFS rate of 57.8%, and patients with active brain metastases had a 12-month PFS rate of 59.6% and a 12-month central nervous system PFS rate of 60.1%.
In the cohort of patients without brain metastases at baseline, the primary endpoint was overall response rate (ORR). The confirmed ORR was 62.7%, which comprised a complete response rate of 9.5% and a partial response rate of 53.1%. This response rate, Lin noted, was in line with previous trials examining T-DXd in this setting.
The safety profile was consistent with previous reports, with no new safety signals reported. However, Lin acknowledged that interstitial lung disease and pneumonitis remained important safety risks.
The invited discussant Cristina Saura, from Vall d’Hebron University Hospital in Barcelona, said, “Until today, the preferred treatment option for patients in the second line with active brain metastases was the tucatinib, trastuzumab, and capecitabine combination. After today’s presentation, I believe the preferred option for the second-line treatment should now be trastuzumab deruxtecan, regardless of whether the patient has active brain metastases or not.”