Interview with Lucia Del Mastro, MD, Department of Internal Medicine and Medical Specialties, School of Medicine, University of Genova, Italy.
Q. Your recent paper in the European Journal of Cancer (EJC 136 (2020) 43-51) on the GIM2 trial is clearly the result of a considerable effort at national level, with many contributing centres all over Italy. Can you tell us more about GIM (Italian Breast Group)?
A. Founded in 2002, the Gruppo Italiano Mammella (GIM group) is the most important italian cooperative group for academic research on breast cancer. Nearly 100 oncologic Italian centres are involved in the group and the number of patients enrolled each year in clinical trial is about 1.000. The research carried out by GIM group in mainly focused on randomized clinical trials and observational/registers studies on both early and metastatic breast cancer. The results of some of these trials (published on Lancet 2015; Lancet oncology, Jama 2011; Jama 2015) were practice-changing and helped to modify national and international guidelines.
Q. To enrol over 1.000 patients exclusively diagnosed with luminal A and B breast cancer must have been not easy. Are you happy with the numbers in terms of statistical significance?
A. The main trial published in Lancet 2015 enrolled 2091 node-positive patients in 3 years and more than 1000 of these patients were luminal A or B. Although the analysis published on EJC 2020 is an unplanned subgroup analysis, the results help to clarify the role of chemotherapy in these two breast cancer subtypes: patients with luminal B node-positive cancer have a worse prognosis compared to luminal A patients and they seem to benefit from dose-dense adjuvant chemotherapy. On the other hand the benefit of this treatment seems to be absent in the subtype (luminal A) in which the efficacy of chemotherapy is still controversial.
Q. Even after your paper it looks still unclear what to do with patients belonging to the Luminal B-like cohort. Would you personally choose dose-dense adjuvant chemotherapy in your clinical practice?
A. Available data indicate that luminal B breast cancer patients have a bad prognosis. In my clinical practice node positive luminal B breast cancer patients do receive dose-dense chemotherapy with sequential anthracycline-taxane regimen, as indicated by main national and international guidelines.
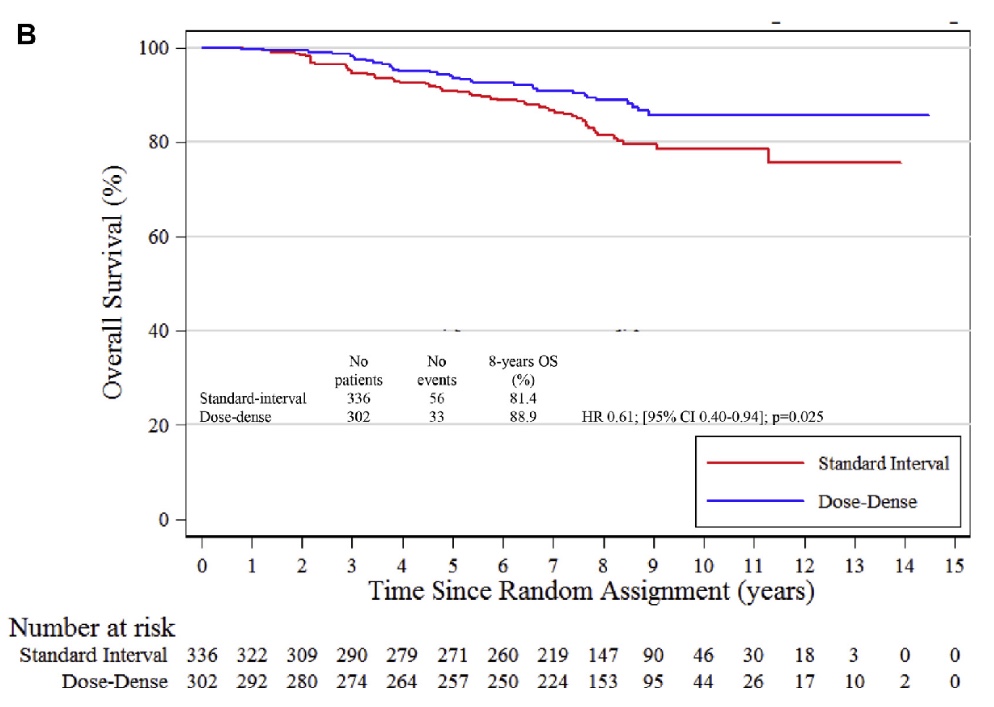
Q. Which is the essential message of your paper and which illustration expresses better?
A. The paper confirms that dose-dense chemotherapy is more efficacious compared to standard interval chemotherapy in high risk breast cancer patients like patients with Luminal B cancer. Dose-dense chemotherapy is associated with a 8% absolute increase in 8-year overall survival and a relative decrease of the risk of death of 39%. Dose dense chemotherapy may be spared in luminal A breast cancer patients.
Q. You belong to the leading group of physician researchers on breast cancer in your country. Do you think that national trials have still a role to play in our globalized world?
A. The GIM group carried out academic trials in which the main topic is not to test new drugs but to test new strategies to improve both efficacy and quality of life of breast cancer patients. These type of trials are more easily carried out at national level, considering that the available budget for these trials is generally restricted. Therefore, it is difficult to involve a large number of centres, especially outside of Italy. Despite the trials are at a “national” levels, they did contribute to modify clinical practice and international guidelines. Moreover, all the GIM trials contribute to the Oxford Early Breast Cancer Trialists cooperative Group meta-analyses.









